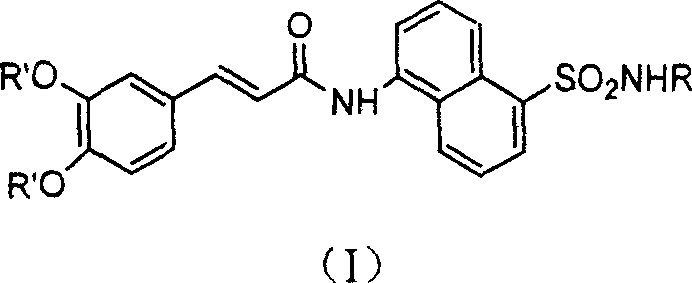
Coffee acyl naphthalene sulfonamides compound and method for preparing the same and anti HIV conformity enzyme action
A technology of naphthalenesulfonamide and caffeamide, which is applied in the field of caffeoylnaphthalenesulfonamide compounds and their preparation, and achieves the effects of simple preparation method, favorable industrial production and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
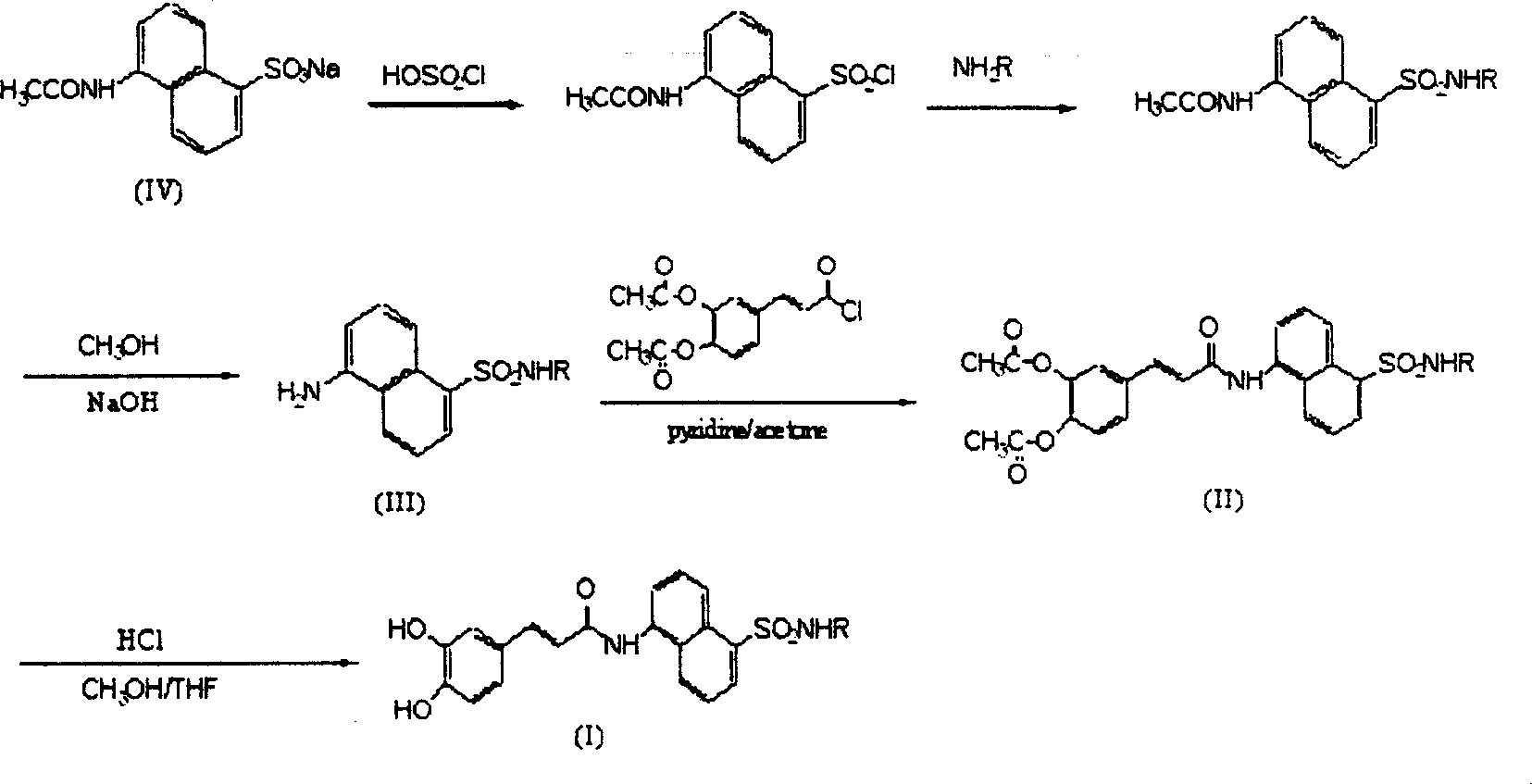
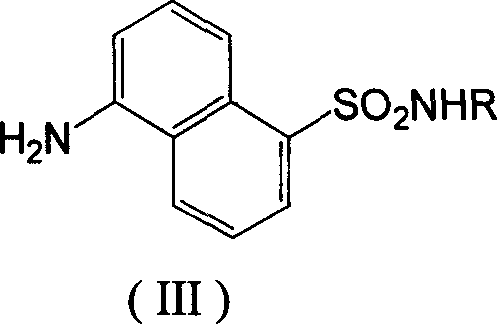
[0027] Example 1: Preparation of 5-(N-phenylamino)-sulfonyl-1-naphthylamine (III)
[0028] Add 3 mL (45 mmol) of chlorosulfonic acid to sodium 5-(acetylamino)-1-naphthalenesulfonate (0.55 g, 2 mmol), stir and react at room temperature for 3 hours, slowly add the reactant dropwise to 40 mL of ice water, and stir , filtered the precipitate, dried to obtain a purple solid, added 3 mmol of aniline, reacted at room temperature for 3 hours, added 40 mL of water, adjusted the pH to 6 with dilute hydrochloric acid, precipitated, filtered, and recrystallized with ethanol to obtain brown crystals. Add this brown crystal (1.57mmol) to a mixed solution of 5mol / L sodium hydroxide (5mL) and methanol (3mL), stir and react at 70°C overnight, cool to room temperature, adjust the pH to 6 with 1mol / L hydrochloric acid, and precipitate out , filtered, drained, and recrystallized from ethanol / water to obtain 5-(N-phenylamino)-sulfonyl-1-naphthylamine, yellow crystals, yield 72%, mp: 170~172°C, R ...
Embodiment 2
[0039] Example 2. Preparation of N-[[5-[(N-phenyl)-amino]-sulfonyl]-1-naphthyl]-3,4-diacetylcaffeamide (II)
[0040] Dissolve 5-(N-phenylamino)-sulfonyl-1-naphthylamine (0.72mmol) in 5mL of acetone, add 5mL of 3,4-diacetylcaffeoyl chloride (0.25g, 0.82mmol) in acetone, and mix well , add 1 mL of pyridine, stir at room temperature for 40 hours, distill off the solvent under reduced pressure, add water and stir, there is precipitation, ethanol / water recrystallization, N-[[5-[(N-phenyl)-amino]-sulfonyl] -1-naphthyl]-3,4-diacetyl caffeamide, yield 63%, mp: 206~208 ℃, Rf=0.4 (ethyl acetate / petroleum ether 1:1); IR (KBr, cm -1 ): 3314, 3070, 1763, 1738, 1658, 1597, 1531, 1497, 1216, 1153, 906~693; 1 H-NMR (DMSO): 10.74(s, 1H), 10.34(s, 1H) 8.61(d, 1H, J=8.6Hz), 8.39(d, 1H, J=8.5Hz), 8.26(d, 1H, J=7.2Hz), 7.97(t, 1H, J=6.9Hz), 7.76(t, 1H, J=8.2Hz), 7.68~7.59(m, 4H), 737(d, 1H, J=8.2Hz) , 7.17~7.02 (m, 5H), 6.93 (t, 1H, J=7.4Hz), 2.31 (s, 6H). IC 50 >100ug / ml. Made by the same m...
Embodiment 3
[0050] Example 3. Preparation of N-[[5-[(N-phenyl)-amino]-sulfonyl]-1-naphthyl]-caffeamide (I)
[0051] Add N-[[5-[(N-phenyl)-amino]-sulfonyl]-1-naphthyl]-3,4-diacetylcaffeamide (2.0mmol) with methanol (2mL), tetrahydrofuran (2mL ) and concentrated hydrochloric acid (1mL), stirred and reacted at 60°C for 0.5 hours, cooled to room temperature, added distilled water (20mL), extracted with ethyl acetate (20mL×2), washed with saturated brine, dried over magnesium sulfate, and reduced Evaporate under pressure to remove solvent, recrystallize petroleum ether / ethyl acetate to obtain N-[[5-[(N-phenyl)-amino]-sulfonyl]-1-naphthyl]-caffeamide, yield 75% , mp: 256~258°C, Rf=0.5 (ethyl acetate / petroleum ether 2:1); IR (KBr, cm -1 ): 3346, 3250, 3037, 1664, 1598, 1516, 1493, 1284, 1147, 927~687; 1 H-NMR(DMSO): 10.69(s, 1H), 10.16(s, 1H), 9.53(s, 1H), 9.23(s, 1H), 8.59(d, 1H, J=8.8Hz), 8.39(d , 1H, J=8.5Hz), 8.24(d, 1H, J=6.3Hz), 7.95(d, 1H, J=6.9Hz), 7.74(t, 1H, J=7.7Hz), 7.67(t, 1H , ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
| Mp | aaaaa | aaaaa |
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com