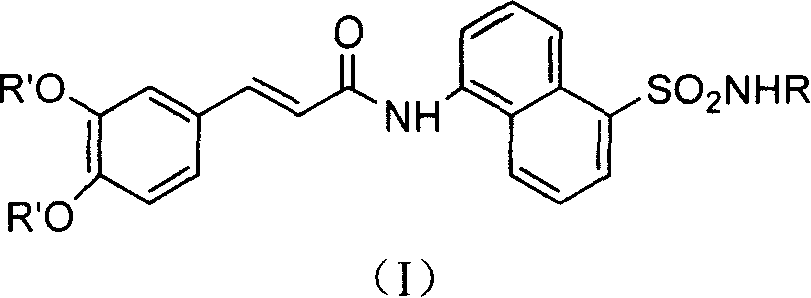
Coffee acyl naphthalene sulfonamides compound and method for preparing the same and anti-HIV conformity enzyme action
A technology of caffeamide and compound, applied in the field of caffeoyl naphthalene sulfonamide compound and its preparation, achieving the effect of high yield, good application prospect and large output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
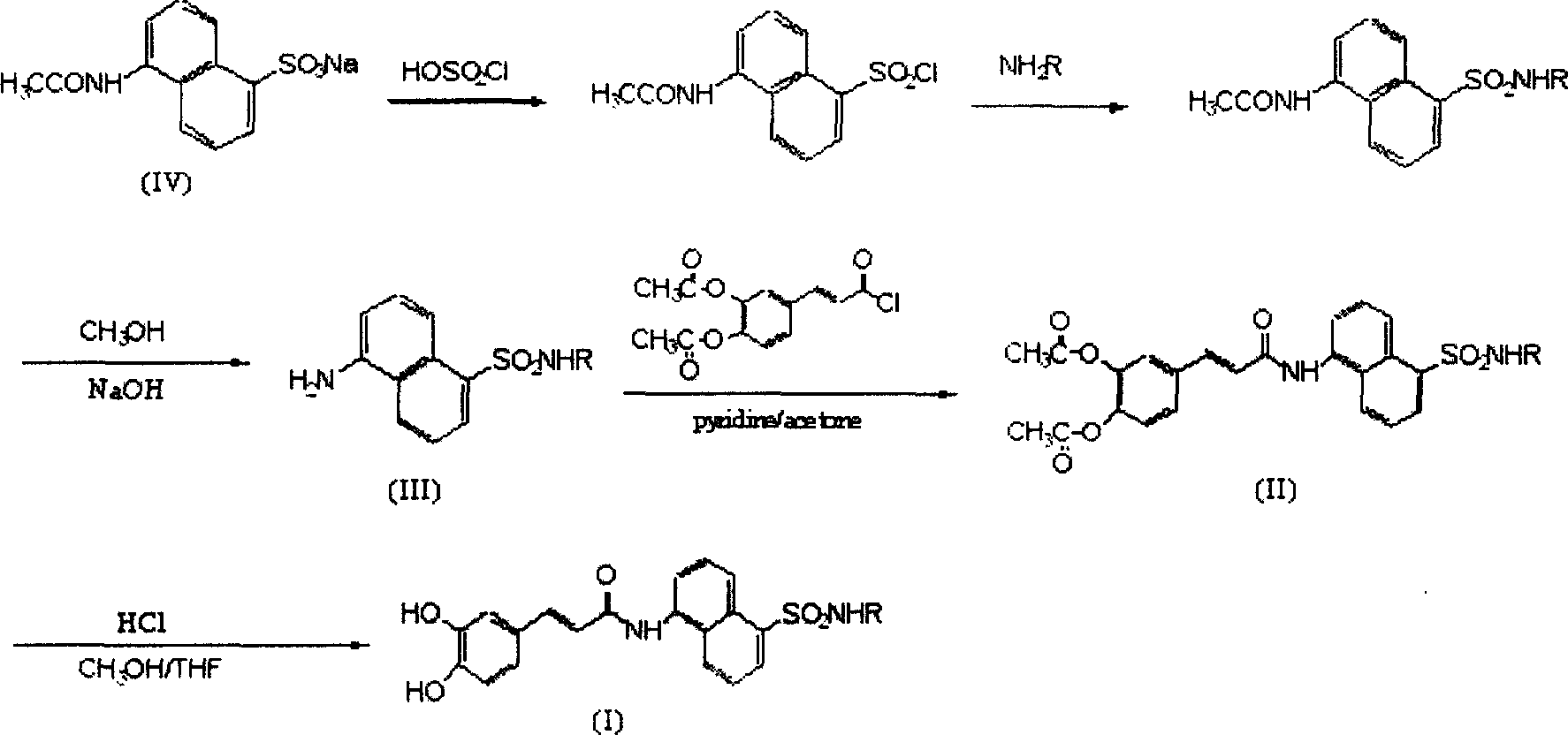
[0026] Example 1: Preparation of 5-(N-phenylamino)-sulfonyl-1-naphthylamine (III)
[0027] Add 3 mL (45 mmol) of chlorosulfonic acid to sodium 5-(acetylamino)-1-naphthalenesulfonate (0.55 g, 2 mmol), stir and react at room temperature for 3 hours, slowly add the reactant dropwise to 40 mL of ice water, and stir , filtered the precipitate, dried to obtain a purple solid, added 3 mmol of aniline, reacted at room temperature for 3 hours, added 40 mL of water, adjusted the pH to 6 with dilute hydrochloric acid, precipitated, filtered, and recrystallized with ethanol to obtain brown crystals. Add this brown crystal (1.57mmol) to a mixed solution of 5mol / L sodium hydroxide (5mL) and methanol (3mL), stir and react at 70°C overnight, cool to room temperature, adjust the pH to 6 with 1mol / L hydrochloric acid, and precipitate out , filtered, drained, and recrystallized from ethanol / water to obtain 5-(N-phenylamino)-sulfonyl-1-naphthylamine, yellow crystals, yield 72%, mp: 170~172°C, R ...
Embodiment 2
[0038] Example 2. Preparation of N-[[5-[(N-phenyl)-amino]-sulfonyl]-1-naphthyl]-3,4-diacetylcaffeamide (II)
[0039] Dissolve 5-(N-phenylamino)-sulfonyl-1-naphthylamine (0.72mmol) in 5mL of acetone, add 5mL of 3,4-diacetylcaffeoyl chloride (0.25g, 0.82mmol) in acetone, and mix well , add 1 mL of pyridine, stir at room temperature for 40 hours, distill off the solvent under reduced pressure, add water and stir, there is precipitation, ethanol / water recrystallization, N-[[5-[(N-phenyl)-amino]-sulfonyl] -1-naphthyl]-3,4-diacetyl caffeamide, yield 63%, mp: 206~208 ℃, Rf=0.4 (ethyl acetate / petroleum ether 1:1); IR (KBr, cm -1 ): 3314, 3070, 1763, 1738, 1658, 1597, 1531, 1497, 1216, 1153, 906~693; 1 H-NMR (DMSO): 10.74(s, 1H), 10.34(s, 1H) 8.61(d, 1H, J=8.6Hz), 8.39(d, 1H, J=8.5Hz), 8.26(d, 1H, J=7.2Hz), 7.97(t, 1H, J=6.9Hz), 7.76(t, 1H, J=8.2Hz), 7.68~7.59(m, 4H), 737(d, 1H, J=8.2Hz) , 7.17~7.02 (m, 5H), 6.93 (t, 1H, J=7.4Hz), 2.31 (s, 6H). IC 50 >100ug / ml.
[0040] Made by t...
Embodiment 3
[0050] Example 3. Preparation of N-[[5-[(N-phenyl)-amino]-sulfonyl]-1-naphthyl]-caffeamide (I)
[0051] Add N-[[5-[(N-phenyl)-amino]-sulfonyl]-1-naphthyl]-3,4-diacetylcaffeamide (2.0mmol) with methanol (2mL), tetrahydrofuran (2mL ) and concentrated hydrochloric acid (1mL), stirred and reacted at 60°C for 0.5 hours, cooled to room temperature, added distilled water (20mL), extracted with ethyl acetate (20mL×2), washed with saturated brine, dried over magnesium sulfate, and reduced Evaporate under pressure to remove solvent, recrystallize petroleum ether / ethyl acetate to obtain N-[[5-[(N-phenyl)-amino]-sulfonyl]-1-naphthyl]-caffeamide, yield 75% , mp: 256~258°C, Rf=0.5 (ethyl acetate / petroleum ether 2:1); IR (KBr, cm -1 ): 3346, 3250, 3037, 1664, 1598, 1516, 1493, 1284, 1147, 927~687; 1 H-NMR(DMSO): 10.69(s, 1H), 10.16(s, 1H), 9.53(s, 1H), 9.23(s, 1H), 8.59(d, 1H, J=8.8Hz), 8.39(d , 1H, J=8.5Hz), 8.24(d, 1H, J=6.3Hz), 7.95(d, 1H, J=6.9Hz), 7.74(t, 1H, J=7.7Hz), 7.67(t, 1H , ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com