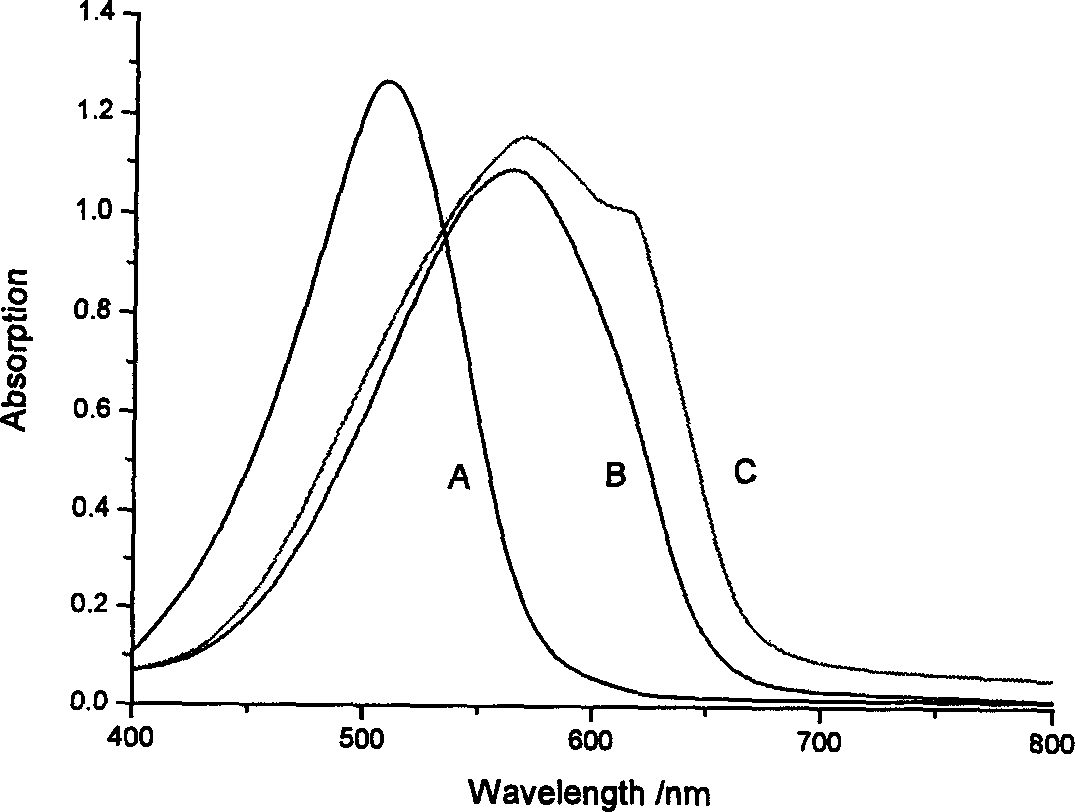
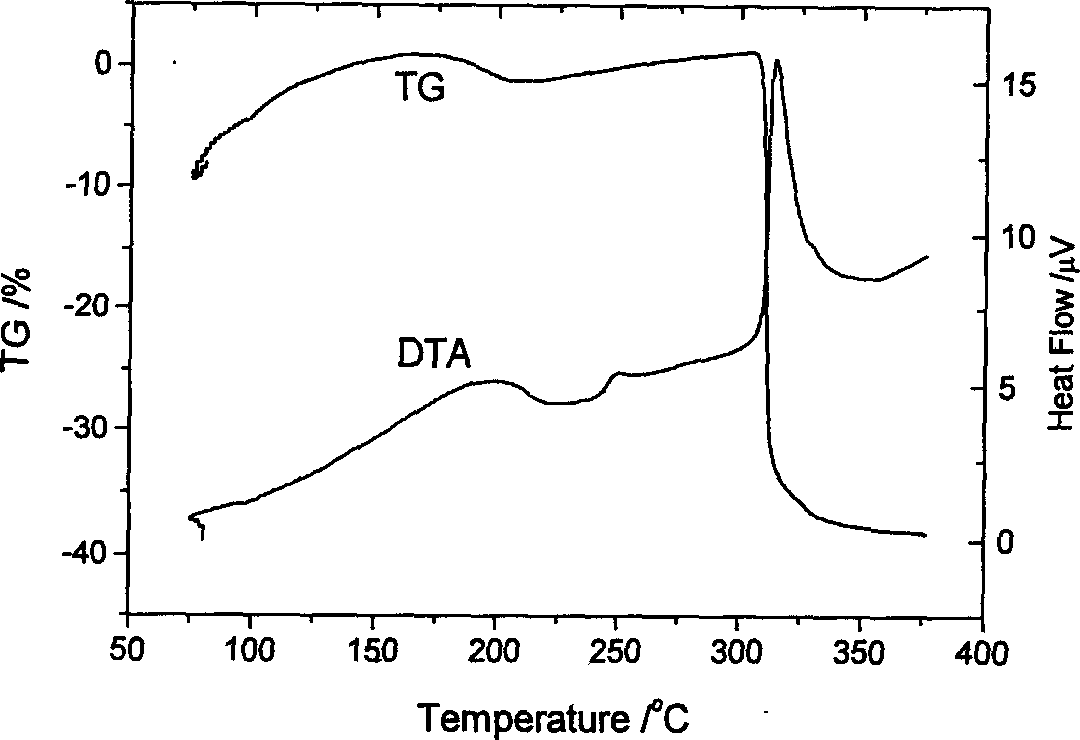
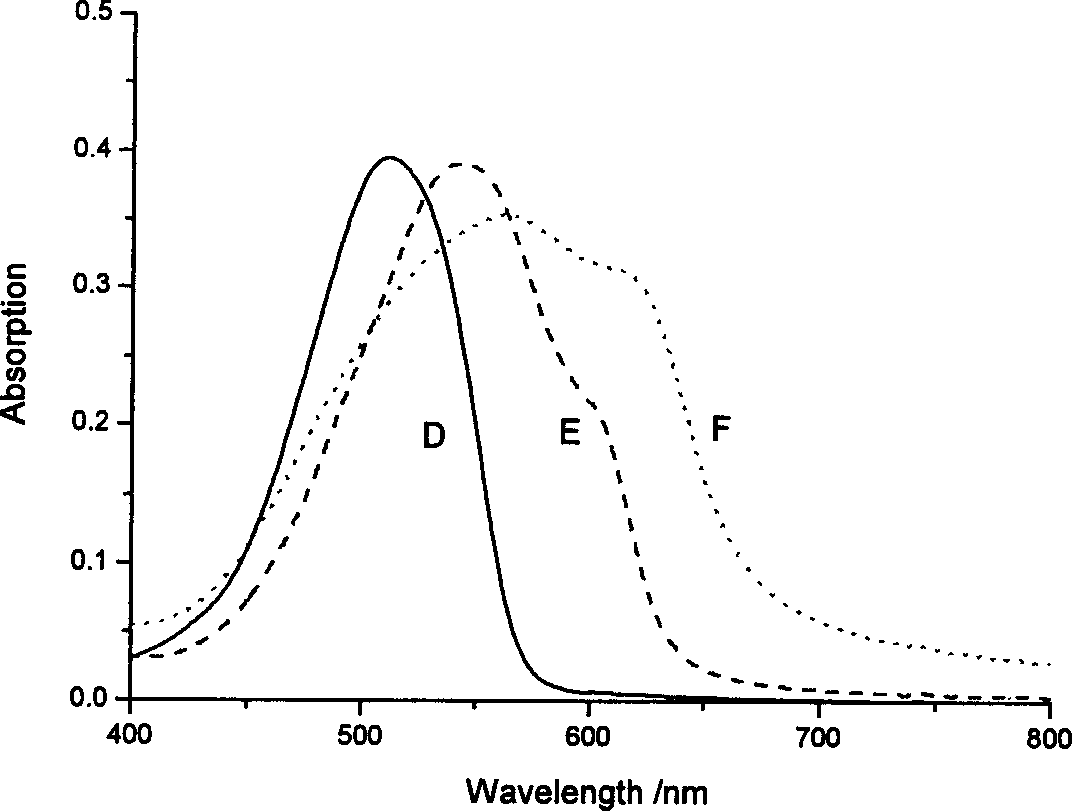
Heterocyclic azo metal chelate compound, their preparation and use
A metal chelate, heterocyclic azo technology, which is applied in the recording/reproducing, instrument, recording information storage and other directions by optical methods, can solve the problems of difficult coupling reaction control, reduce the purity of azo compounds, etc., and achieve good optical performance. Effects of recording properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 150mg (1.5mmol) of 2-aminothiazole was dissolved in 4ml of 85% phosphoric acid, cooled to 0-5°C with an ice-salt bath, and 109mg of solid sodium nitrite was added at this temperature, kept for 2 hours, and then a small amount of urea was added to Destroy excess nitrous acid.
[0027] Dissolve 477mg of the coupling component 3-(p-toluenesulfonamido)-N,N-diethylaniline (1.5mmol) in a mixture of 3mL water and 3mL acetic acid, and add 2g sodium acetate to form a buffer. The solution was cooled to 0-5° C. with an ice-salt bath, and the above diazonium salt solution was slowly added dropwise and kept for 2 hours. After filtering and drying, the crude dye was 365mg, and the yield was 85.1%. The dye was purified by column chromatography on neutral alumina (eluent was dichloromethane). Azo dye MS(C 20 h 23 N 5 S 2 o 2 ):M + =429. Elemental analysis (C 20 h 23 N 5 S 2 o 2 ): Calculated: C, 55.92; H, 5.40; N, 16.30; S, 14.93%; Found: C, 55.49; H, 5.51; N, 16.40; S, 1...
Embodiment 2
[0033]1.3g (5mmol) of 2-amino-5-bromothiazole hydrogen bromide was dissolved in 10ml of 85% phosphoric acid, cooled to 0-5°C with an ice-salt bath, and 360mg of solid sodium nitrite was added at this temperature, and kept for 2 hours, and then add a small amount of urea to destroy excess nitrous acid.
[0034] Dissolve 825 mg of coupling component 3-diethylaminophenol (5 mmol) in 15 ml of 95% ethanol, and cool the reaction system to 0-5° C. with an ice-salt bath. The above diazonium salt solution was slowly added dropwise, and the reaction was stirred at this temperature for 3 hours. Then 20 ml of water was added, and the reaction was stirred for 3 hours. The reaction mixture was left overnight, at which time the temperature of the system was allowed to rise to room temperature. Filter, wash with water, and dry to obtain 1.53 g of crude dye, with a yield of 86.2%. The dye was separated and purified by neutral alumina column chromatography (the eluent was chloroform). Dye M...
Embodiment 3
[0040] 200mg (2.0mmol) of 2-aminothiazole was dissolved in 4ml of 85% phosphoric acid, cooled to 0-5°C with an ice-salt bath, and 145mg of solid sodium nitrite was added at this temperature, kept for 2 hours, and then a small amount of urea was added to Destroy excess nitrous acid.
[0041] Dissolve 518mg of coupling component 3-(N,N-diethyl)amino-4-methoxybenzenesulfonic acid (2.0mmol) in 4mL of water and 4mL of acetic acid mixture, add 3g of sodium acetate to make a buffer . The solution was cooled to 0-5° C. with an ice-salt bath, and the above diazonium salt solution was slowly added dropwise and kept for 2 hours. After filtering and drying, the crude dye was 575mg, and the yield was 77.7%. The dye was purified by column chromatography on neutral alumina (eluent was dichloromethane). Its structural formula is
[0042]
[0043] 120mg (0.32mmol) of azo dye was dissolved in 10ml of ethanol solvent, and nickel acetate (0.64mmol) dissolved in 2ml of water was added to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com