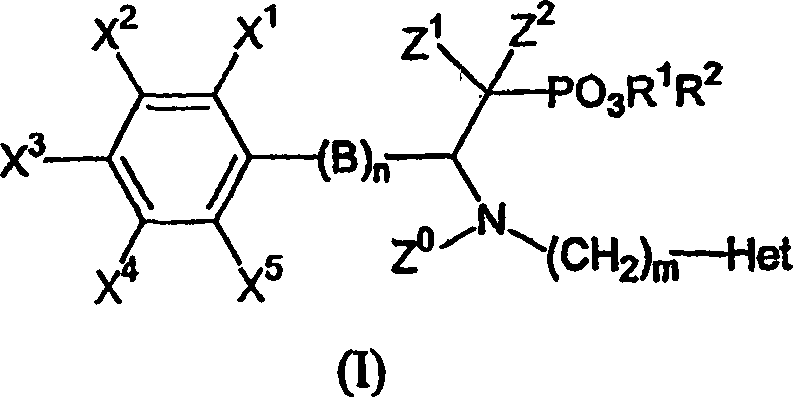
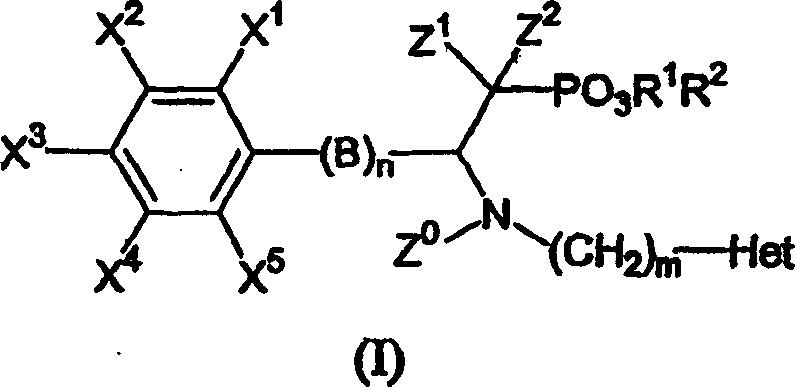
Beta-substituted befa-aminoethyl. phosphonates compound
A technology of diethyl ethyl phosphonate and compounds, applied in the field of substituted aminoethyl phosphonate compositions, can solve the problems of not being able to reduce plasma Lp concentration, failing to meet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1 β-(3,5-dimethoxy-4-hydroxyphenyl)-β-[N-(3-pyridyl)-amino]-ethylphosphonic acid diethyl ester
[0095]
[0096] Add imidazole (14.8g, 217.6mmol) in portions to the stirred syringaldehyde (12.0g, 65.83mmol) and tert-butyldimethylsilyl chloride (14.9g, 98.9mmol) in 80ml of N, N-dimethyl The mixture in formamide (DMF) was continuously stirred at room temperature for 3 hours. The mixture was poured into water kept at 0°C, and 25% ammonium hydroxide solution was added thereto until the pH reached 7. The aqueous phase was extracted with dichloromethane and the organic phase was dried over magnesium sulfate. Evaporation of the solvent gave an oil which was purified by column chromatography (silica gel, eluent: CH 2 Cl 2 ). 16.4 g (84%) of the pure fraction were obtained as a solid, mp = 74-76°C.
[0097] 10.6g (35.8mmol) of 4-(tert-butyldimethylsilyloxy)-3,5-dimethoxybenzaldehyde, 3.36g (35.8mmol) of 3-aminopyridine and a catalytic amount of p-toluenesulfonate...
Embodiment 2
[0113] β-(4-Hydroxy-3-methoxy-5-methylphenyl)-β-[N-(3-pyridyl)-amino]-ethylphosphonic acid diethyl ester
[0114]
[0115] With 35.0g (0.21mol) 4-hydroxyl-3-methoxy-5-methylbenzaldehyde (mp=98-100 ℃), 19.8g (0.21mmol) 3-aminopyridine and catalytic amount of p-toluenesulfonic acid (about 10 mg) of the mixture was dissolved in 150 ml of toluene in a flask connected to a Dean-Stark apparatus and refluxed for 6 hours. Quantitative formation of the Schiff base is indicated by the formation of an equivalent amount of water. The toluene solution was evaporated to dryness to give 51 g (100%) of the crude imine. To this material and a DMF solution (300 ml) of tert-butyldimethylsilyl chloride (47.6 g, 0.32 mol) was added imidazole (28.7 g, 0.42 mol) in three portions, and the resulting mixture was stirred at room temperature for 6 hours . The mixture was poured into ice water, neutralized with 25% ammonia solution and finally with CH 2 Cl 2 extraction. dry (MgSO 4 ) organic ph...
Embodiment 3
[0132] β-(4-Hydroxy-3-methoxy-5-methylphenyl)-β-[N-(3-(2,6-dimethyl)pyridyl))-amino]-ethylphosphonic acid Dimethyl ester
[0133]
[0134] A mixture of 4-hydroxy-3-methoxy-5-methylbenzaldehyde (15.0 g, 90.4 mmol), 3-amino-2,6-lutidine (11.0 g, 90.36 mmol) and 5 mg TsOH was dissolved Reflux for 7 hours in 150 ml of toluene in a flask connected to a Dean-Stark apparatus. Toluene was evaporated to dryness to give 24.4 g (100%) of an orange oil which was used directly in the next step. To a solution of this material (24.4g, 90.4mmol) and tert-butyldimethylsilyl chloride (20.4g, 135.6mmol) in DMF (150ml) was added imidazole (12.3g, 180.7mmol) and the resulting mixture was dissolved in Stir at room temperature for 6 hours. Pour the mixture into ice water, neutralize with 25% ammonia solution, and finally 2 Cl 2 extraction. Evaporation of the dry solvent gave the crude Tbs-protected imine as a brown oil (28 g, 81%).
[0135] To 120 ml of anhydrous THF was added n-butyllithi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com