Method for preparing sulfone by catalytic oxidation of thioether
A technology for oxidizing sulfur and sulfide, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of low conversion rate and high cost, and achieve the effects of easy separation, reduced pollution and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Catalyst preparation:
[0017] 14.4mmol FeCl 3 and 8.0 mmol MnCl 2 4H 2 O was dissolved in 20 mL deionized water, 0.4 mmol RuCl 3 and 1.2mmol Cu(NO 3 ) 2 The mixed aqueous solution was added to the above solution while stirring; the obtained mixed solution was slowly added to 40 mL of 124.2 mmol NaOH aqueous solution while stirring. After the addition, continue to stir the obtained slurry and raise the temperature to 110°C. After stirring for another 2 hours, stop stirring and let it cool naturally; After one hour, be cooled to room temperature, grind and promptly make 1.75g black solid catalyst MnFe 1.8 Cu 0.15 Ru 0.05 o 4 .
Embodiment 2
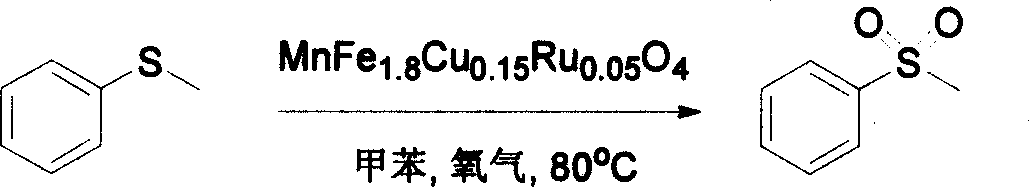
[0019] Oxidation of sulfide anisole:
[0020] The oxidation reactions of sulfide anisole were carried out in glass tubes. The reaction tube is heated by an oil bath. 2mmol of sulfide anisole, 0.3g of MnFe 1.8 Cu 0.15 Ru 0.05 o 4 The catalyst and 5 mL of toluene solvent were mixed in the reaction tube in advance. Oxygen was provided by commercially available balloons filled with oxygen. The reaction products were detected by Shimadzu GC-14C gas chromatography, and naphthalene was used as an internal standard. The reaction temperature was 80°C. The reaction formula is as follows:
[0021]
[0022] After reacting for 12 hours under the above conditions, the conversion rate of sulfide anisole was 100%, and the selectivity of methyl phenyl sulfone was over 99%.
Embodiment 3
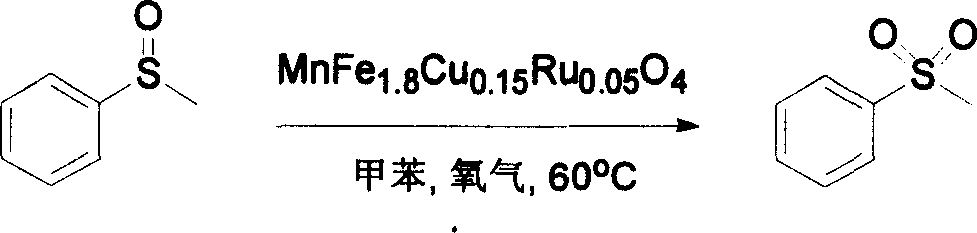
[0024] Oxidation of methyl phenyl sulfoxide:
[0025] The oxidation reactions of methyl phenyl sulfoxide were carried out in glass tubes. The reaction tube is heated by an oil bath. 2mmol of methyl phenyl sulfoxide, 0.3g of MnFe 1.8 Cu 0.15 Ru 0.05 o 4 The catalyst and 5 mL of toluene solvent were mixed in the reaction tube in advance. Oxygen was provided by commercially available balloons filled with oxygen. The reaction products were detected by Shimadzu GC-14C gas chromatography, and naphthalene was used as an internal standard. The reaction temperature was 60°C. The reaction formula is as follows:
[0026]
[0027] After reacting for 6 hours under the above conditions, the yield of the product methyl phenyl sulfone was over 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com