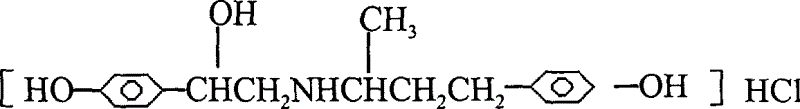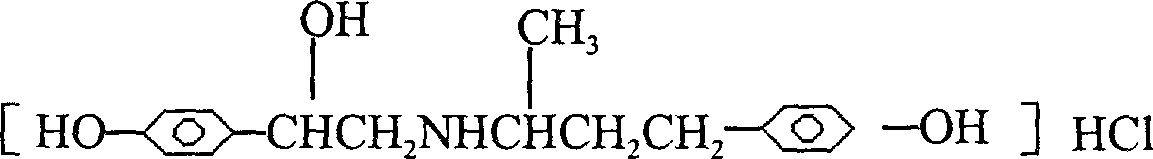Novel process for preparing ractopamine from p-hydroxyacetophenone and raspberry ketone
A technology of p-hydroxyacetophenone and ractopamine, which is applied in the field of preparation of organic compounds, can solve the problems of low yield of N-alkylation reaction, high market price, and influence on yield, and achieves less by-products and better synthesis Simple steps and good quality results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 300 milliliters of carbon tetrachloride, 25.0 grams of p-hydroxyacetophenone in a 500 milliliter four-neck round bottom reaction flask with magnetic stirring, reflux condenser, chlorine conduit and thermometer, heat to reflux (temperature is 85 ℃), pass Chlorine gas was added to react, and the end point was determined by spotting on a thin-layer chromatographic plate. After the reaction, cool down, add sodium bicarbonate to neutralize, filter, concentrate and crystallize the filtrate, and obtain white crystals after drying.
[0038] Yield: 25.1 Yield: 85%
[0039] Melting point: 130-132°C
[0040] Elemental Analysis: C 8 h 7 o 2 CL M.W.: 170.6
[0041] Theoretical value: C56.32 H4.14 Cl20.78
[0042] Analytical value: C56.09 H4.06 Cl21.12
Embodiment 2
[0044] Add 100 milliliters of 10% sodium carbonate aqueous solution, 100 milliliters of ethyl acetate, 8.2 grams (50 mmol) of 1-methyl-3-(4-hydroxyphenyl)-propylamine and 10.0 g (60 mmol) of -chloro-p-hydroxyacetophenone, stirred vigorously at room temperature. In the first few minutes, all the reactants were dissolved into a brownish-red solution. After about one hour, a large amount of white precipitates began to be produced, and the reaction was completed after about 4 hours.
[0045] Filter with suction, wash with ethyl acetate and distilled water three times each, and drain.
[0046] Transfer the filter cake into a 500ml flask, add 100ml of water, add concentrated hydrochloric acid dropwise until pH = 2, heat to reflux, stir to acidify, and cool to crystallize.
[0047] Yield: 6.0 g Yield: 40.8%
[0048] Melting point: 105-107°C
[0049] Elemental Analysis: C 18 h 22 o 3 CL M.W.: 335.8
[0050] Theoretical value: C64.37 H6.60 N4.17 Cl10.56
[0051] Analytical value:...
Embodiment 3
[0053] Add 30.0 grams (90 mmol) of the white powdery product obtained in Example 2 in a 1000 milliliter hydrogenation reactor, 3.0 grams of Raney nickel, 500 milliliters of absolute ethanol, and hydrogenate at 8Mpa at 140°C until no hydrogen is absorbed.
[0054] After the hydrogenation is completed, filter, and the filtrate is distilled under reduced pressure to remove ethanol, and dried to obtain a light yellow solid product, which is Ractopamine Hydrochlide.
[0055] Melting point: 137-139°C
[0056] Yield: 29.7 g Yield: 99%
[0057] Elemental Analysis: C 18 h 23 o 3 CL M.W.: 337.83
[0058] Theoretical value: C63.99 H7.16 N4.15 Cl10.49
[0059] Analytical value: C63.69 H7.03 N4.08 Cl10.36
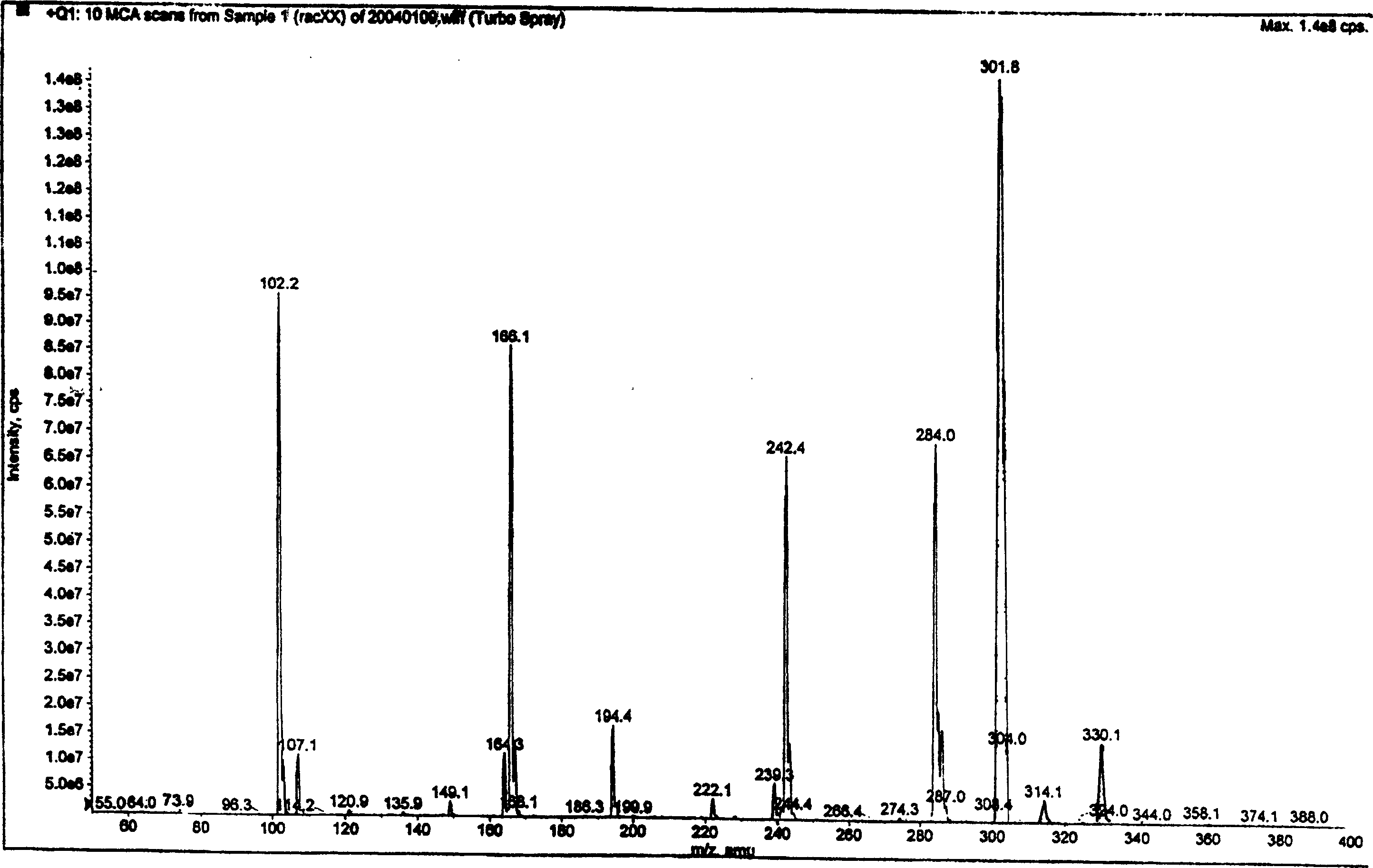
[0060] (The results of product mass spectrometry are shown in the attached figure 1 :)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com