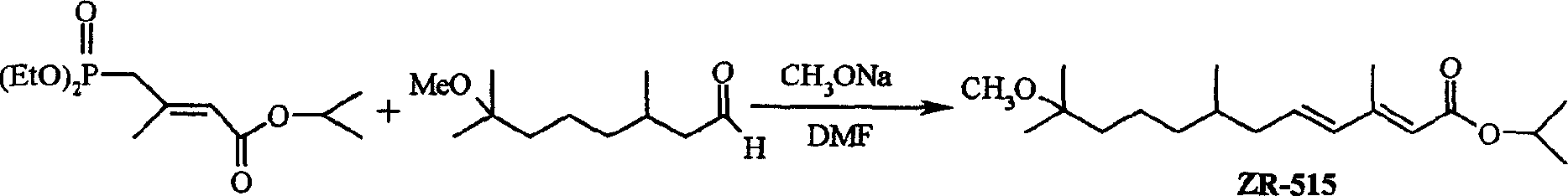
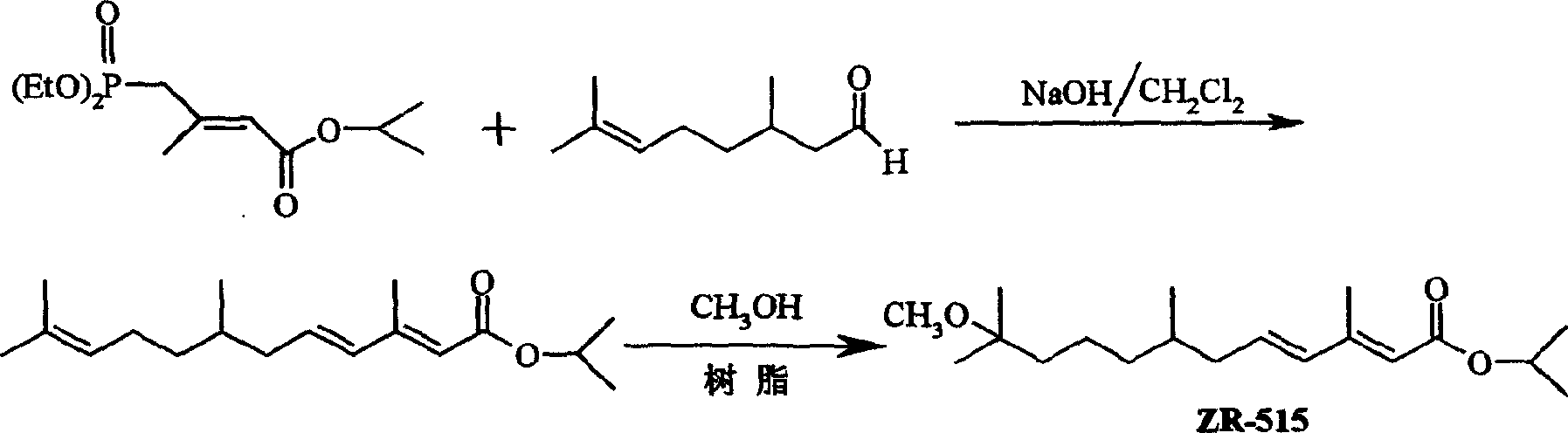
Synthesis process for analogue 2R-515 of juvenile hormone
A ZR-515, juvenile hormone technology, applied in hormone peptides, animal/human proteins, specific peptides, etc., can solve the problems of expensive raw materials and reagents, environmental friendliness, unsuitable for large-scale preparation, harsh reaction conditions, etc. High target ether selectivity, lower synthesis cost, mild reaction conditions and environmental protection effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The commercially available strongly acidic cation exchange resin was treated with 2M hydrochloric acid solution at a ratio of liquid to solid mass ratio of 8 for 10 hours at 50°C, filtered and washed with deionized water until there was no chloride ion (silver nitrate solution test), at 80 °C for 24 hours to obtain a hydrogen-form strongly acidic cation exchange resin, which is ready for use.
[0025] Add 150mL of 50% aqueous sodium hydroxide solution, 120mL of dichloromethane, and tetrabutylammonium bromide (6.0g) into a 250ml three-necked flask in sequence, and add citronellal (15.6g) and dichloromethane dropwise under stirring at room temperature. A mixed solution of methane 50mL and 4-(diethoxyphosphoryl)-3-methyl-2-butenoic acid isopropyl ester (27.8g), after the dropwise addition was completed, the reaction was stirred at room temperature for 30h, and dissolved in 2M hydrochloric acid neutralize, separate liquid, extract the aqueous phase with dichloromethane (100...
Embodiment 2
[0028] Treat the commercially available strongly acidic cation exchange resin with 3M hydrochloric acid solution at a ratio of liquid to solid mass ratio of 6 for 30 hours at room temperature, filter and wash with deionized water until there is no chloride ion (tested by silver nitrate solution), and store at 90°C Dry for 12 hours to obtain a hydrogen-type strongly acidic cation exchange resin, which is set aside.
[0029]Citronellal (15.6g), 4-(diethoxyphosphoryl)-3-methyl-2-butenoic acid isopropyl ester (30.6g), dichloromethane 140mL, tetrabutylammonium bromide ( 5.0 g) was added to a 1L three-necked flask in turn, and 200 mL of 40% aqueous sodium hydroxide solution was added dropwise at room temperature under stirring. After the addition was completed, the reaction was stirred at room temperature for 36 h, neutralized with 2M hydrochloric acid until neutral, and separated. The aqueous phase was extracted with dichloromethane (100mL×3), and the organic phases were combined, ...
Embodiment 3
[0032] Treat the commercially available strongly acidic cation exchange resin with 1M hydrochloric acid solution at a ratio of liquid to solid mass ratio of 10 at room temperature for 20 hours, filter and wash with deionized water until there is no chloride ion (tested by silver nitrate solution), and store at 60°C Dry for 48 hours to obtain a hydrogen-type strongly acidic cation exchange resin, which is set aside.
[0033] Citronellal (15.6g), 4-(diethoxyphosphoryl)-3-methyl-2-butenoic acid isopropyl ester (25.0g), dichloromethane 120mL, tetrabutylammonium bromide ( 4.0g) and 180mL of 45% aqueous sodium hydroxide solution were added to a 500mL three-necked flask, stirred at room temperature for 24h, neutralized to neutral with 2M hydrochloric acid, separated, the aqueous phase was extracted with dichloromethane (100mL×3), and the organic phase, washed with water, washed with saturated sodium bicarbonate, washed with water, washed with saturated brine, dried over anhydrous mag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com