Tumor suppressor, coded protein and application thereof
A tumor suppressor and protein technology, applied in the field of application of the gene and encoded protein, can solve the problem of unconfirmed autophosphorylation combination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Acquisition of the mNTKL-BP1 gene
[0094] 1. Construction of "bait" plasmid pDB-Leu-mNTKL
[0095] The cDNA sequence encoding the mouse mNTKL protein was obtained as a positive clone when using the N-terminus of the human ALC1 gene as a bait protein to screen mouse embryos and fetal brain cDNA libraries, and was fused in the pPC86 vector (ProQuest of U.S. Invitrogen Corporation) TM Yeast two-hybrid system) on the AD 3' end, digested and ligated by restriction enzyme SalI / NotI, two fragments of 0.9kb and 2.2kb, firstly connect the 2.2kb fragment with the double-digested recovery fragment of the vector, and then pass SalI Single enzyme digestion, dephosphorylation treatment, insertion of a 0.9kb fragment, identification of the insertion direction, and completion of subcloning the entire coding sequence of mNTKL into the SalI-NotI cloning site of the vector pDB-Leu (Invitrogen Company), thereby constructing a "bait" Plasmid pDB-Leu-mNTKL.
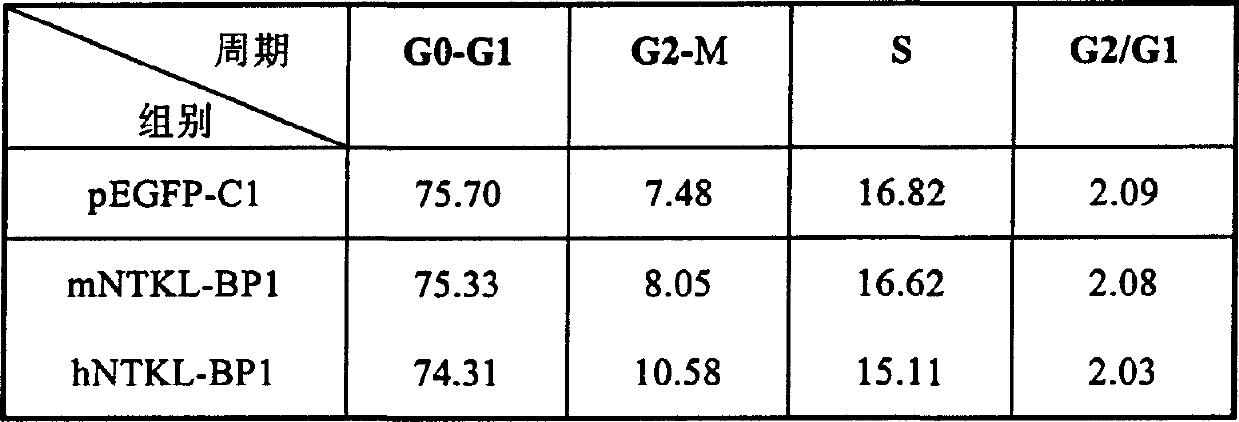
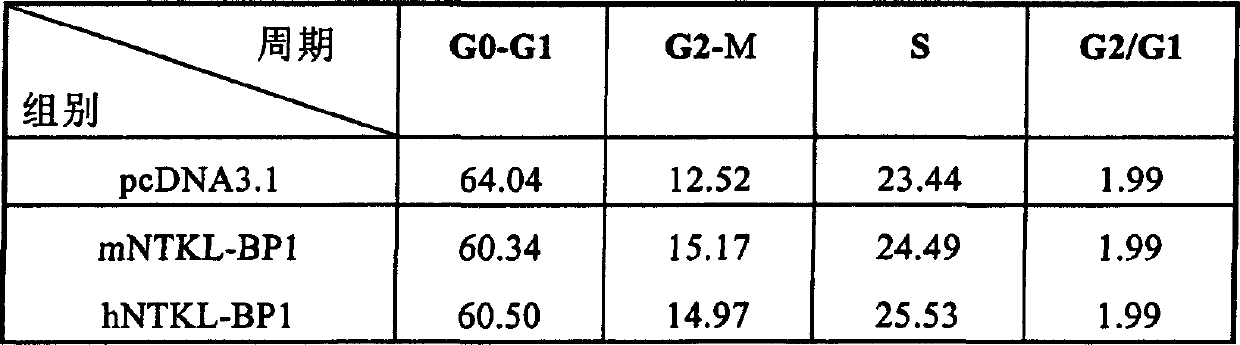
[0096] 2. Autonomous activati...
Embodiment 2
[0102] Acquisition of human hNTKL-BP1 gene
[0103] The following primers were synthesized,
[0104] Forward primer number: HBF,
[0105] Sequence: 5'-CGG ATG AGC TGG GCA GCA GTG TTG G-3' (SEQ ID NO: 5)
[0106] Reverse primer number: HBR,
[0107] Sequence: 5'-ACC AGA ACT TCA TGT GGC CAA AGC A-3' (SEQ ID NO: 6)
[0108] Using Polymerase Chain Reaction (Polymerase Chain Reaction, PCR) technology, using the Marathon-ready cDNA library of human fetal brain tissue (purchased from U.S. Clontech Company) as template amplification, PCR reaction was performed with Advantage TM 2 Polymerase enzyme, reaction conditions according to the American Clontech company Marathon TM cDNA Amplification kit manual operation.
[0109] The amplified product was sequenced to obtain a DNA sequence (SEQ ID NO: 3) containing a complete open reading frame (open reading frame, ORF) with unknown function. According to the inventor's research on the function of the protein encoded by this gene, it w...
Embodiment 3
[0111] Sequence analysis of hNTKL-BP1 gene
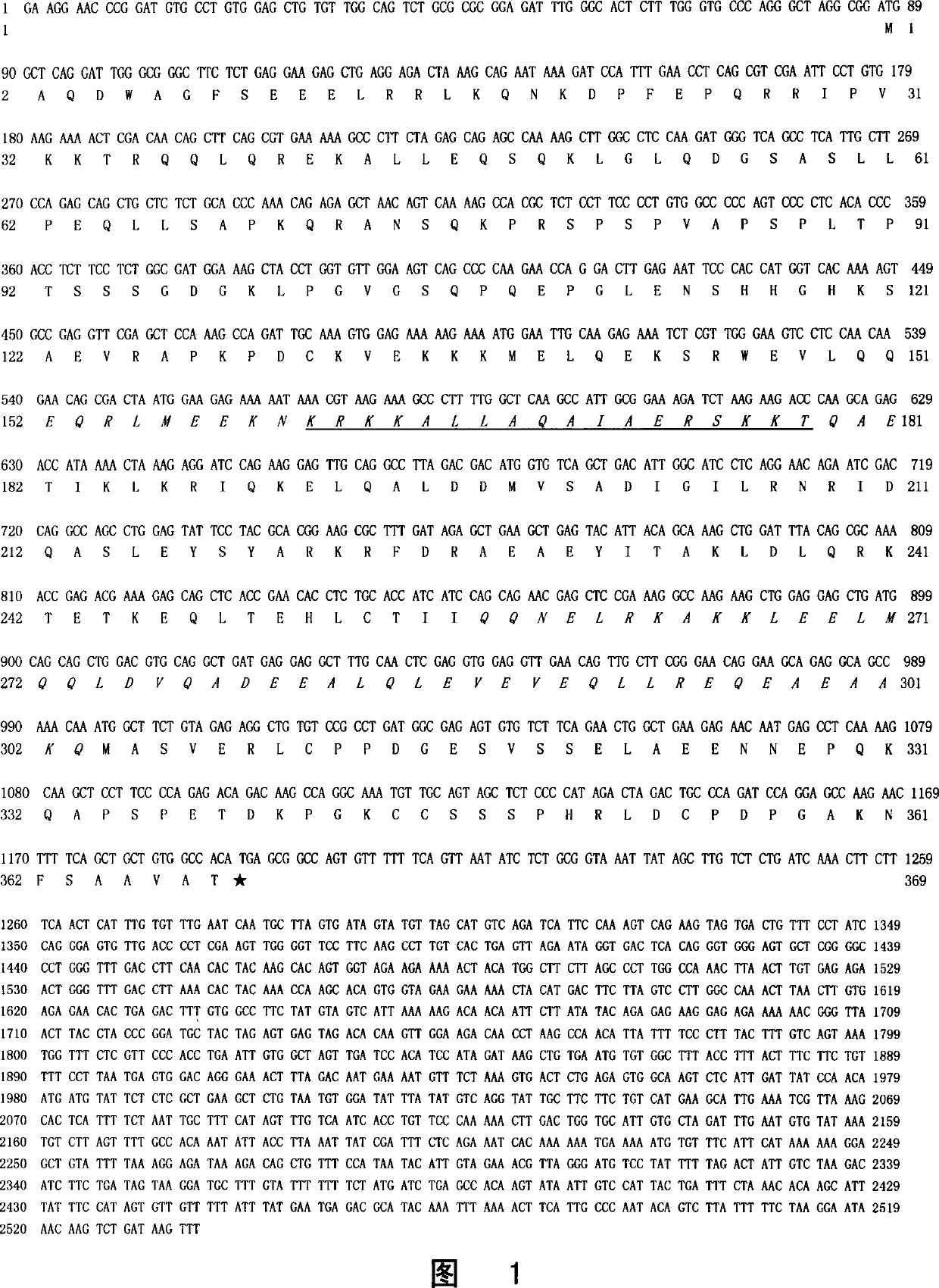
[0112] Using the primers designed by us, the human hNTKL-BP1 gene DNA sequence cloned by PCR reaction is 1214bp in length (SEQ ID NO: 3). The protein coding frame sequence is 21-1202, encoding a protein with 394 codons (Fig. 3 and SEQ ID NO: 4), including two coiled coil domains. Compared with the homologous DNA sequence XM_044455 registered in GenBank, the full length of XM_044455 is 2576bp, encoding a deduced open reading frame (from 21bp-1205bp) containing 394 amino acids, and its corresponding protein sequence accession number is: XP_044455.
[0113] The DNA sequence homology of the human hNTKL-BP1 gene and the mouse mNTKL-BP1 gene reaches 80%, and the amino acid sequence of the protein reaches 89%. However, the N-terminus of human hNTKL-BP1 protein encodes 22 more amino acids than that of mouse (Fig. 2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com