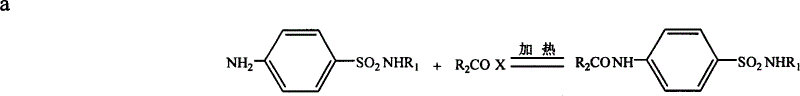Method for preparing sulfonamide compound liposome and its formulation
A compound and sulfonamide technology, applied in the field of medicine, can solve the problem that sulfonamide drugs are not easily encapsulated into liposomes, and achieve the effects of high encapsulation efficiency and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of indapamide liposome injection
[0035] 1. Add an appropriate amount of indapamide to an appropriate amount of anhydrous pyridine at a certain temperature to dissolve, add an excess of lauroyl chloride under magnetic stirring, and after a certain period of reaction, add a certain concentration of hot acid water to react the excess lauroyl chloride. Add a strong base to dissolve it, then use an acid to precipitate it once, collect the precipitate, dissolve the precipitate with a certain solvent, and separate lauroyl indapamide by silica gel column chromatography.
[0036] The reaction equation is as follows:
[0037]
[0038] 2. Dissolve a certain amount of lauryl indapamide, phospholipids, cholesterol, and vitamin E with 2ml of ethanol.
[0039] 3. Pour the above solution into a 60°C solution containing 0.2% Ca under magnetic stirring. 2+ in isotonic buffer solution.
[0040] 4. Ethanol was evaporated under reduced pressure.
[004...
Embodiment 2
[0043] Embodiment 2: the preparation of sulfisoxazole liposome gel.
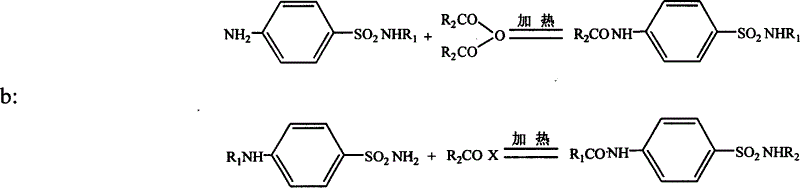
[0044] 1. Dissolve sulfisoxazole in anhydrous pyridine at a certain temperature to dissolve, add excess acetic anhydride under magnetic stirring, after a certain period of reaction, add acid water at a certain temperature to react the excess anhydride, add a strong base to make it Dissolve, then use acid to precipitate once, collect the precipitate, dissolve the precipitate with a certain solvent, and obtain N-acetylsulfaisoxazole with relatively high purity after proper separation.
[0045] The reaction equation is as follows:
[0046]
[0047]2. Dissolve N-acetylsulfisoxazole, phospholipids, cholesterol and vitamin E acetate in absolute ethanol or 95% ethanol and mix well.
[0048] 3. Pour the mixed solution into a 0.5% Ba 2+ in normal saline
[0049] 4. Ethanol was evaporated under reduced pressure.
[0050] 5. Remove Ba by dialysis or adding appropriate amount of EDTA-Na 2+
[0051] 6. Add 1% Ca...
Embodiment 3
[0052] Example 3: Preparation of long-circulating liposome sulfamethazine lyophilized liposome powder injection
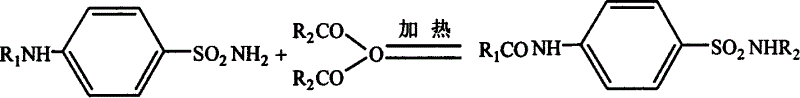
[0053] 1. Dissolve sulfamethazine in anhydrous pyridine at a certain temperature, add excess benzoyl bromide under magnetic stirring, after reacting for a certain period of time, add acid water at a certain temperature to react excess acid anhydride, add strong base to make Dissolve it, and then use acid to precipitate it once, collect the precipitate, dissolve the precipitate with a certain solvent, and obtain N-acetylsulfaisoxazole with relatively high purity after proper separation. Modified in the following way
[0054]
[0055] 2. After the modified drug is properly separated, it can be dissolved in ethanol:ether (v / v, 8:2) with soybean lecithin, appropriate amount of DMPE-PEG20000, cholesterol, and propyl gallate
[0056] 3. Inject the solution containing 0.3% Mg 2+ Acetic acid-sodium acetate buffered saline solution (adjusted isotonic with NaCl) at pH 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com