Antibodies for inhibiting blood coagulation and methods of use thereof
An antibody, inhibited technology, applied in the field of novel human tissue factor antibodies, can solve the problems of unsuitable anticoagulants, TF-binding antibodies do not have binding affinity, etc., achieve excellent anticoagulant activity, reduce coagulation, and inhibit blood coagulation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
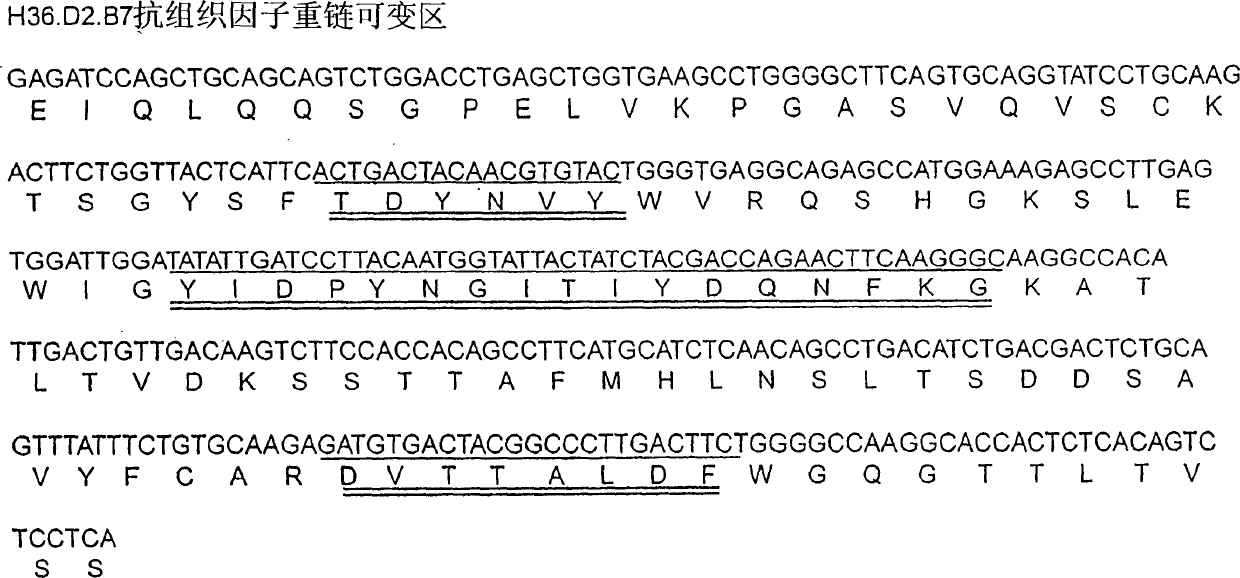
[0252] Embodiment 1-Preparation and Transformation of Anti-rhTF Monoclonal Antibody
[0253] Anti-rhTF monoclonal antibody was prepared as follows.
[0254] A. Immunizations and booster vaccinations
[0255] Five female BALB / c mice were immunized with 10 micrograms each of lipidated purified rhTF. Mice were initially sensitized by intraperitoneal injection with Hunter's Titermax adjuvant. Three final boosters were administered with 0.85% sodium chloride. The booster vaccinations were given at 2, 5.5 and 6.5 months after the initial sensitization. All boosters were given intraperitoneally, but the first dose was given subcutaneously. The final booster vaccination of 20 lines was given 3 days before fusion, and 20 micrograms were given.
[0256] B. Fusion of mouse spleen lymphocytes with mouse myeloma cells
[0257] Lymphocytes from the spleen of rhTF-immunized BALB / c mice were fused to X63-Ag8.653 mouse myeloma cells using PEG1500. After exposure to PEG, cells were incub...
Embodiment 2
[0283] Example 2 - Binding activity of antibodies of the invention
[0284] The antibody of the present invention prepared in Example 1 above was used. The rhTF molecule was expressed in E. coli and purified by immunoaffinity chromatography according to standard methods (see Harlow and Lane, supra, Ausubel et al., supra). The association constant (Ka) and dissociation constant (Kd) of the antibody were determined by ELISA and surface cytoplasmic resonance (i.e. BIACore) assay (see for example Harlow and Lane, supra, Ausubel et al., supra; Altschuh et al., Biochemistry, 31:6298 (1992); and the BIACore method disclosed by Pharmacia Biosensors Inc.). For BIACore assays, rhTF was immobilized on a biosensor chip according to the manufacturer's instructions. Each antibody constant was determined at four antibody concentrations (0.125 nM, 0.25 nM, 0.5 nM and 1 nM).
[0285] Protein concentrations were determined by standard assay assays (M.M. Bradford, Analytical Biochemistry, 72:...
Embodiment 3-F
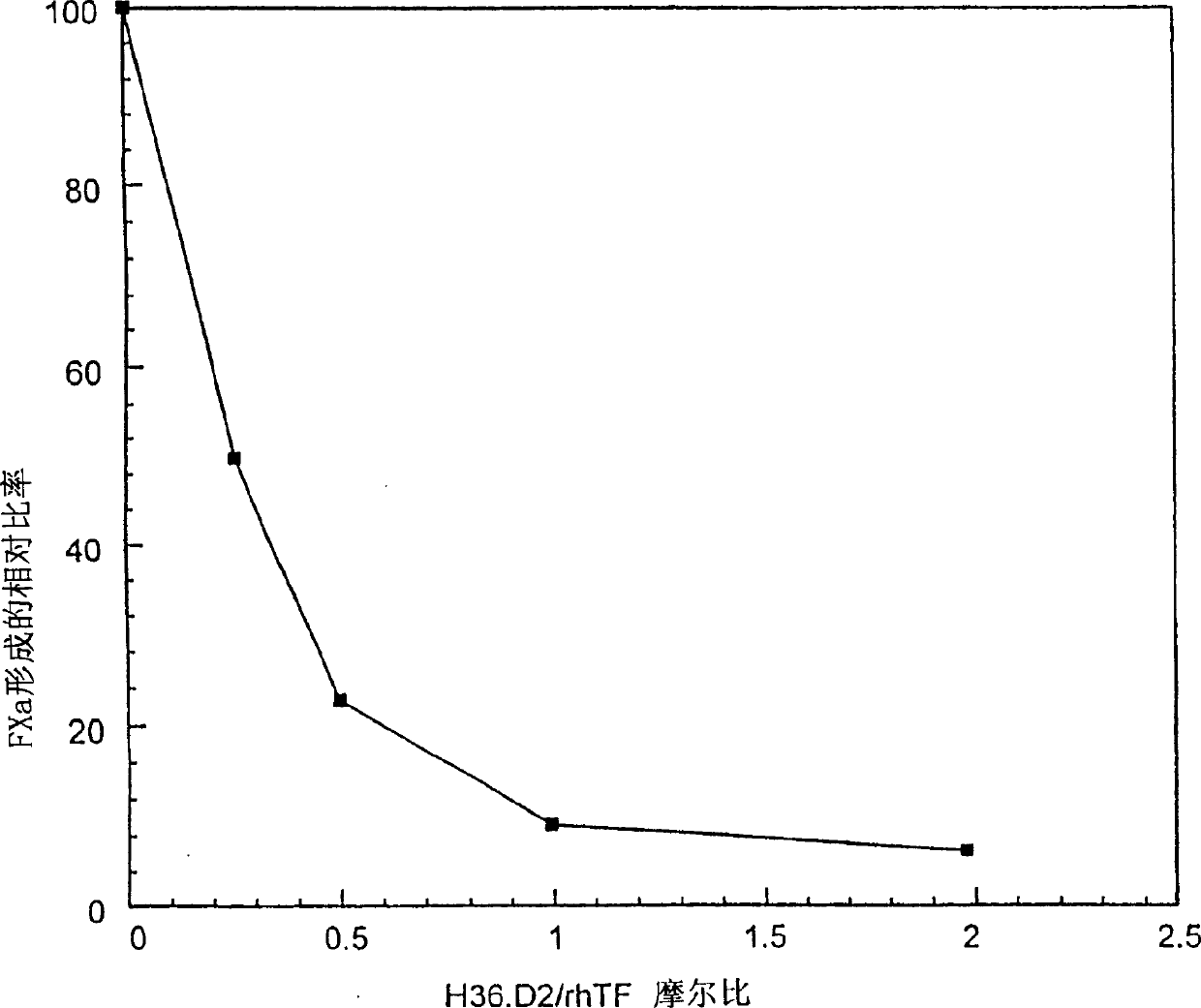
[0287] Example 3-FXa Specific Matrix Assay Analysis
[0288] Typically the experiments described here were performed using rhTF using phosphatidylcholine (0.07 mg / ml) and phosphatidylserine (0.03 mg / ml) in a 70 / 30 w / w ratio in 50 mM Tris-HCl, pH 7.5, 0.1% bovine serum albumin (BSA) lipidation at 37°C for 30 minutes. A stock solution of preformed TF:FVIIa complex was prepared by incubating 5 nM lipidated rhTF and 5 nM FVIIa at 37°C for 30 minutes. TF:FVIIa complexes were aliquoted and stored at -70°C until needed. Purified human factors VII, VIIa and FX were obtained from Enzyme Research Laboratories. The following buffers were used for all FXa and FVIIa assays: 25 mM Hepes NaOH, 5 mM CaCl 2 , 150mM NaCl, 0.1% BSA, pH7.5.
[0289] Monoclonal antibodies were screened for their ability to block TF:VIIa-mediated activation of FX to FXa. Activation of FX is carried out in two sequential steps. In the first step (FX activation), at Ca +2 The conversion of FX to FXa was assaye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com