Conjugated ramification material of fluorine containing anthracene and pyrene at 9th position, preparation method and application
A derivative and conjugated technology, applied in the field of optoelectronic materials, to achieve the effect of simple route, improved device efficiency, and improved injection and transmission capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Synthesis of Trimeric (9-phenyl-9-pyrenylfluorene) ter(9-phenyl-9-pyrenylfluorene) (TPPF)
[0046] (1) 2,7-dibromofluorenone and 2-bromofluorenone.
[0047] Dissolve 10.1 g of 2,7-dibromofluorene in 150 mL of pyridine solution, add 1 mL of benzyltrimethylammonium hydroxide in methnol (40%) (or tetrabutylammonium hydroxide in methanol) to this solution, and then stir with air for 24 hours. Pyridine was removed by distillation under reduced pressure to obtain a reddish brown solid. The solid was recrystallized in ethanol (or column chromatography with ethyl acetate / petroleum ether=10:1) to obtain 9.8 g of yellow solid with a yield of 93%. 1 HNMR (400MHz, CDCl3) δ (ppm): 7.77 (d, J = 1.6Hz, 2H); 7.63 (dd, J = 8.0, 1.6Hz, 2H), 7.39 (d, J = 8.0Hz, 2H).
[0048] Using 2-bromofluorene as raw material, 2-bromofluorenone can be obtained in a similar method with a yield of 93%. 1 HNMR (400MHz, CDCl3) δ (ppm): 7.77 (d, J = 1.6Hz, 1H); 7.66 (dt, J = 7.2Hz, 1.2Hz, 1H);...
Embodiment 2
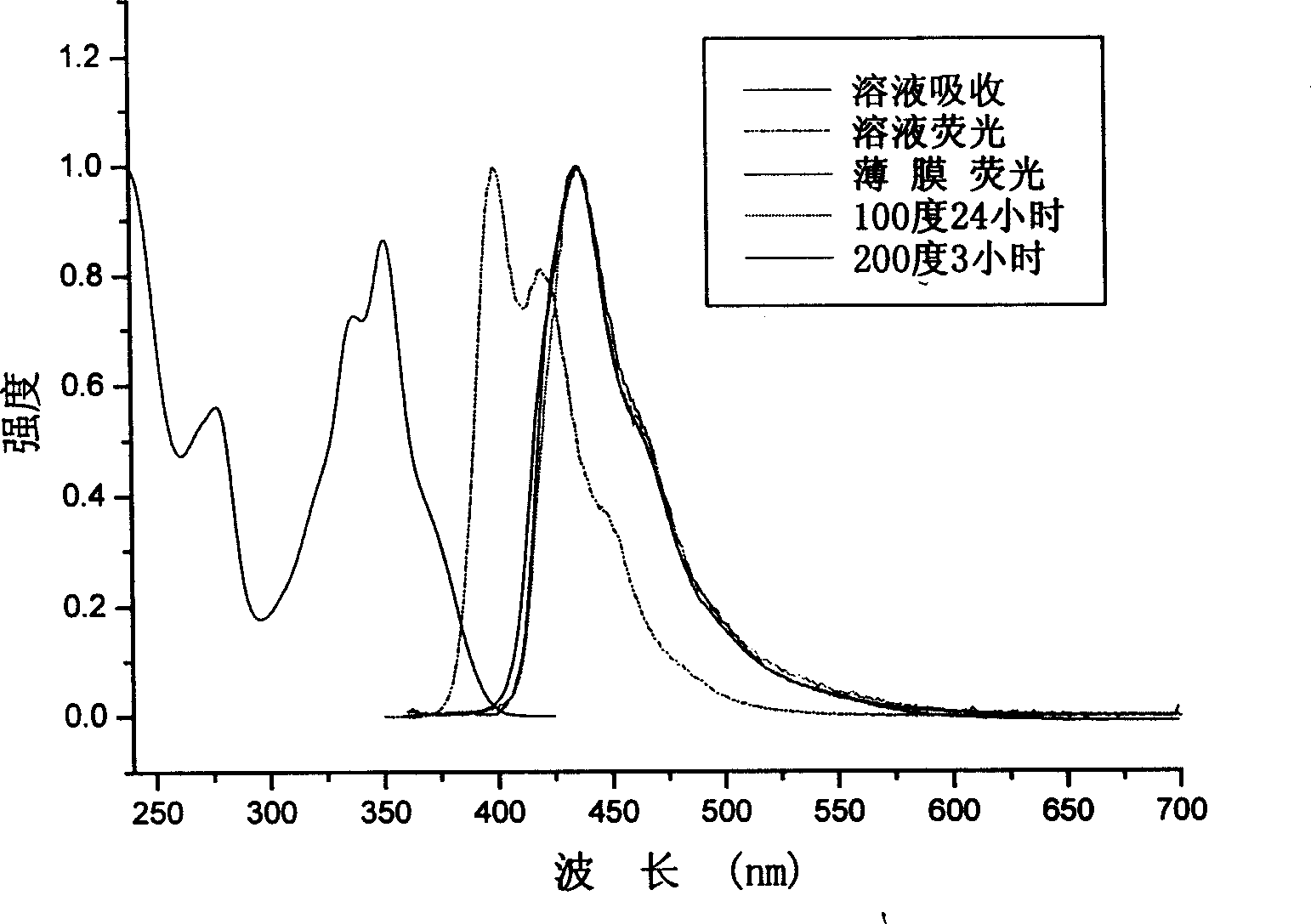
[0060] Embodiment 2: to the ultraviolet absorption spectrum of the trimer TPPF (the product in embodiment 1) that contains anthracene, pyrene fluorene at 9 positions, photoluminescence spectrum, spectral thermal stability and quantum efficiency measurement:
[0061] TPPF was dissolved in dichloromethane dilute solution saturated with nitrogen, and measured by Shimadzu UV-3150 ultraviolet-visible spectrometer and absorption wavelength (351nm). The photoluminescence spectrum of the solid film was carried out through a vacuum-evaporated quartz plate with a film thickness of 300 nm. The fluorescence quantum efficiency of the solution is measured by 10 in cyclohexanone -6 A 9,10-dibenzanthracene solution of M (with a quantum efficiency of 1.0) was used as a standard for measurement.
[0062] The maximum absorption peak of TPPF solution above 300nm is 351nm, and the photoluminescence spectrum has two characteristic absorptions, namely 399 and 419nm.
[0063] The maximum emission w...
Embodiment 3
[0064] Example 3: Synthesis and Spectral Measurement of Trimeric (9-Anthracenyl-9-Phenylfluorene) (TAPF).
[0065] Using a method similar to TAPF, except that pyrene is replaced by anthracene, TAPF can be obtained in similar yield.
[0066] The ultraviolet absorption and photoluminescence spectra can be measured by a method similar to TAPF. The photoluminescence spectrum was measured at the maximum absorption wavelength (355 nm) of ultraviolet absorption.
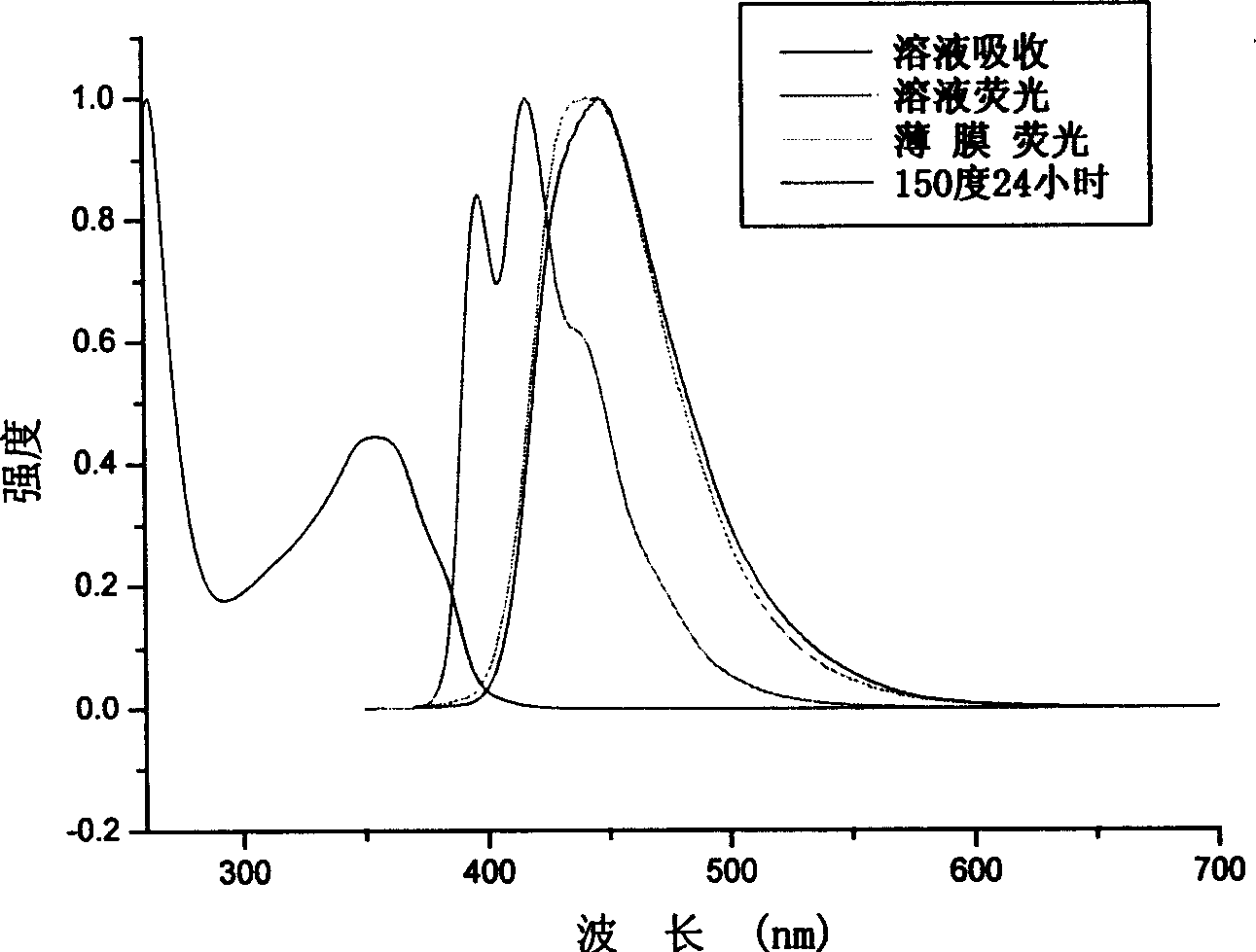
[0067] The maximum absorption peak of TPPF solution above 300nm is 355nm, and the photoluminescence spectrum has two characteristic absorptions, namely 397 and 417nm.
[0068] The maximum emission wavelength of the solid film is 442nm. The solid film was annealed at 150°C for 24 hours under a nitrogen atmosphere, and the spectrum did not change significantly, indicating that the spectral stability was very good due to the good thermal stability. See attached figure 2 .
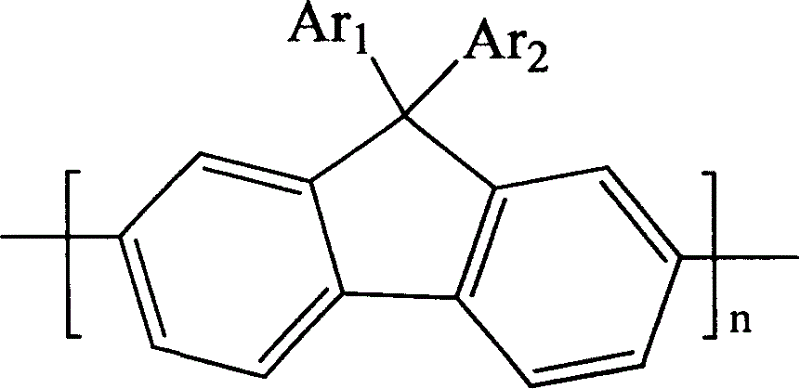
[0069] The structural formula of TAPF is as fol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com