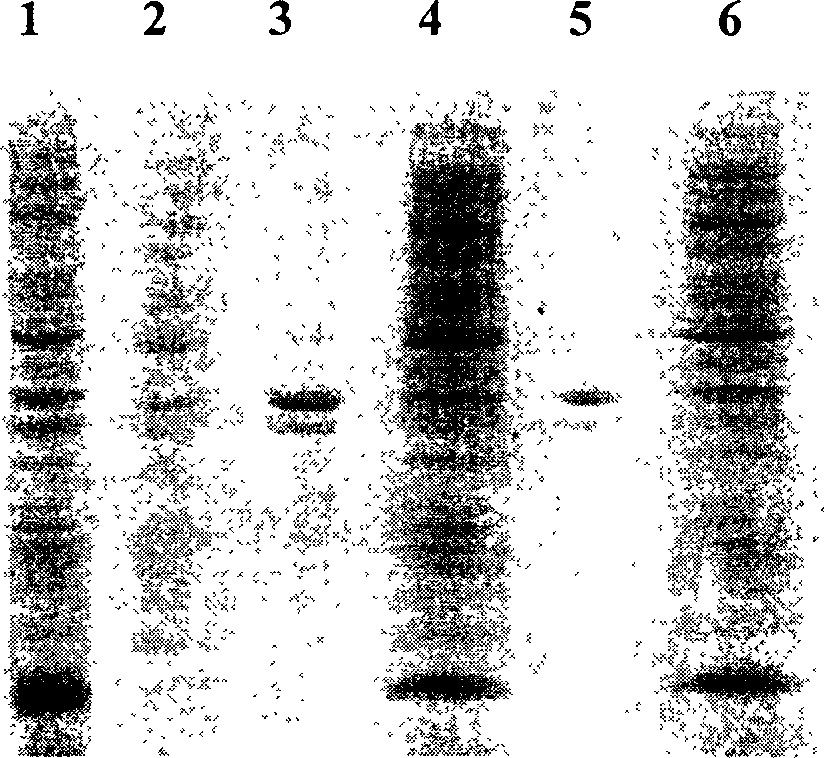Horny cell growth factor mutant with high bioactivity and its preparation process and use thereof
A growth factor, keratinocyte technology, applied in biochemical equipment and methods, botanical equipment and methods, growth factors/inducing factors, etc., can solve problems such as KGF-2 mutants that have not yet been seen, and achieve broad market development and development. Application prospects, reduce scar formation, and delay the effect of skin cell aging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Cloning of embodiment one recombinant human KGF-2 and KGF-2 / STEAcDNA sequence
[0041] 1. Materials
[0042] 1. Strains, vectors and cells
[0043] Both the E.coli BL21 strain and the pET17b prokaryotic expression vector were preserved in our laboratory, and the normal rat tracheal epithelial cells (RTE) were purchased from the Peking Union Medical College Cell Center.
[0044] 2. Enzymes and Reagents
[0045] Human fetal liver cDNA library was purchased from Clontech Company, BamHI, NdeI, Taq DNA polymerase, Pfu DNA polymerase and dNTP were purchased from TaKaRa Company, T4 DNA ligase and mini-plasmid extraction kit were purchased from Promega Company, DNA recovery kit Purchased from Dingguo Biotechnology Company, recombinant human FGFR2β(IIIb) / Fc was purchased from R&D Company, enzyme label plate and 96-well cell culture plate were purchased from Costar Company, TMB was purchased from AMRESCO Company, HRP-labeled secondary antibody was purchased from CHEMICON Compan...
Embodiment 2
[0052] Expression and purification of embodiment two recombinant human KGF-2 and KGF-2 / STEA
[0053] 1. Materials
[0054] Same as embodiment one.
[0055] 2. Methods and Results
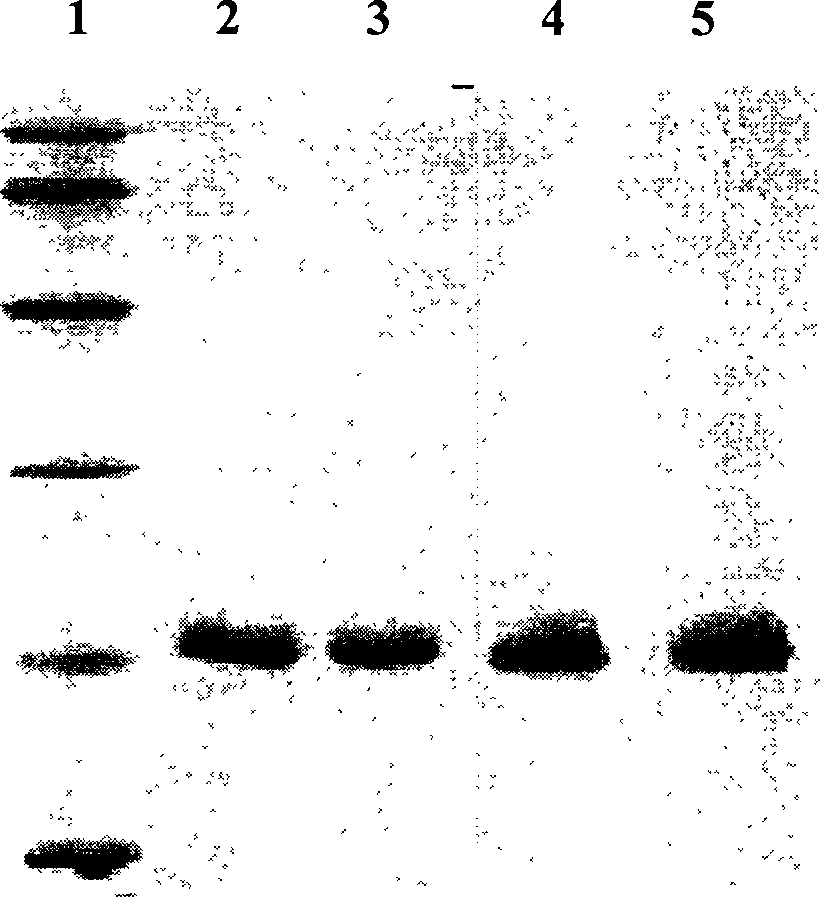
[0056] Pick single-clonal colonies from pET17b / KGF-2 and pET17b / STEA-transformed E. coli BL21 plates, insert them into 50ml of ampicillin-resistant LB medium containing 0.5% glucose, and shake on a shaker at 35°C to OD 600 = 0.6-1.0. Harvest the bacteria and transfer them into 1L ampicillin-resistant RM medium, shake at 35°C to OD 600 =0.5-1.0, IPTG was added to a final concentration of 0.5 mM. After induction of expression for 5 hours, the bacteria were harvested by centrifugation at 8000 rpm at 4°C. The bacterial cell pellet was resuspended in 50 ml of 20 mM PB (pH 7.4, containing 2 mM EDTA) buffer, and ultrasonically disrupted until the solution was transparent. Centrifuge at 10,000 rpm at 4°C, take the supernatant and pass through the SP cation exchange column, and the target protein is el...
Embodiment 3
[0057] The biological activity assay of embodiment three recombinant human KGF-2 and KGF-2 / STEA
[0058] 1. Materials
[0059] Same as embodiment one.
[0060] 2. Methods and results
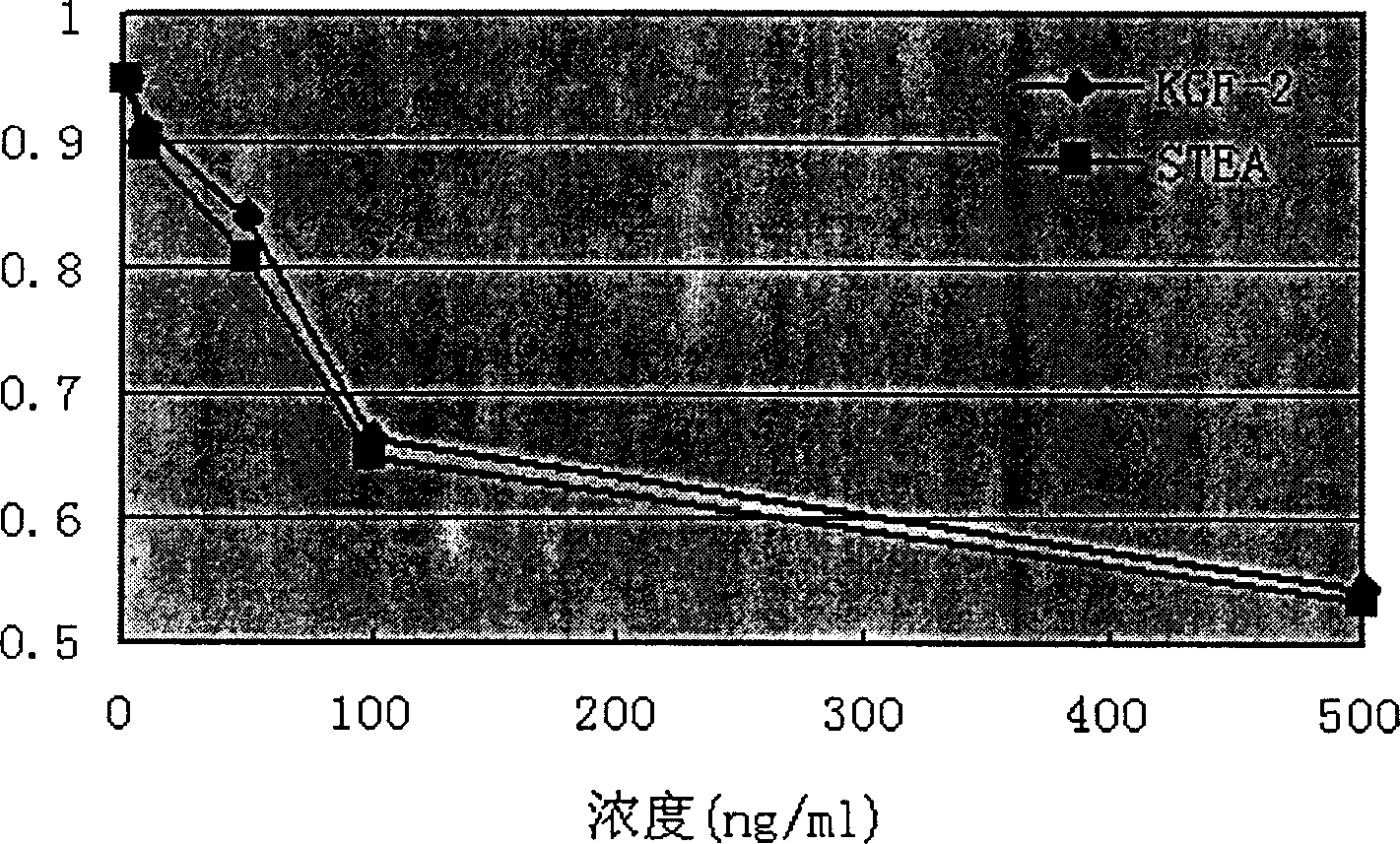
[0061] 1. Detection of receptor binding ability of recombinant human KGF-2 and KGF-2 / STEA
[0062] The receptor binding ability of recombinant human KGF-2 and KGF-2 / STEA was detected by competitive ELISA method, and the specific operation was as follows: the purified recombinant human KGF-2 was coated with diluent (NaCO 3 1.59g, NaHCO 3 2.93g, dilute to 1L with distilled water) to 10μg / ml, add 100μl / well to a 96-well microtiter plate, and coat at 4°C overnight; the next day, use PBS (PH 7.4, NaCl 8g, KH 2 PO 4 0.24g, Na 2 HPO 4 1.44g, KCl 0.2g, distilled water to 1L), wash 3 times, 2min / time; add 200μl 3% skimmed milk powder (3g skimmed milk powder dissolved in 100ml PBS) to each well, block at 37°C for 2h; PBS containing 0.05% Tween20) was washed 3 times, 2 min / time; 100 μl of FGFR2IIIb r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com