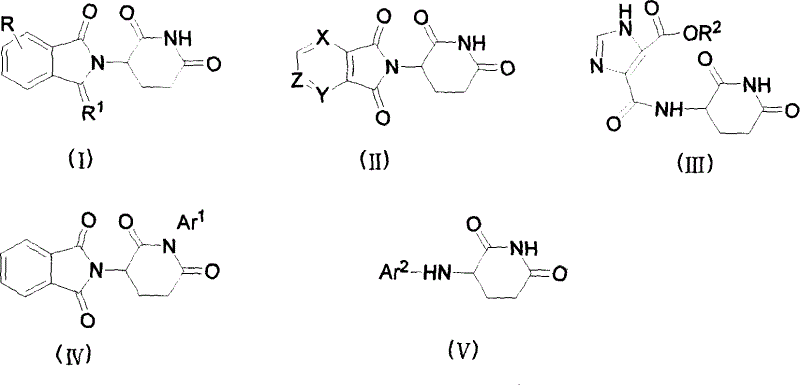
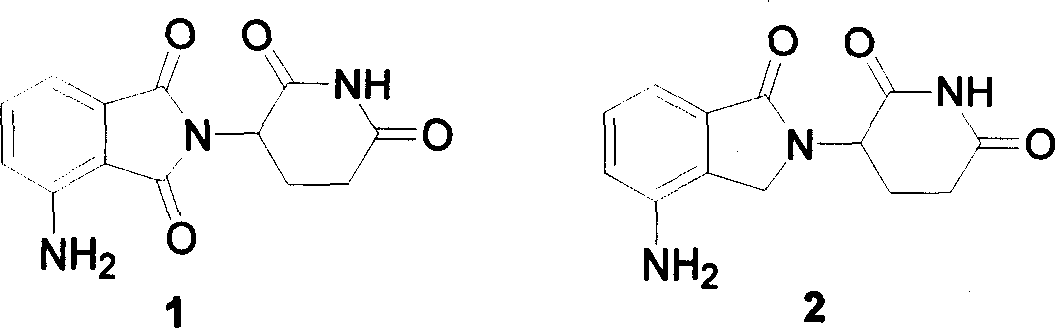
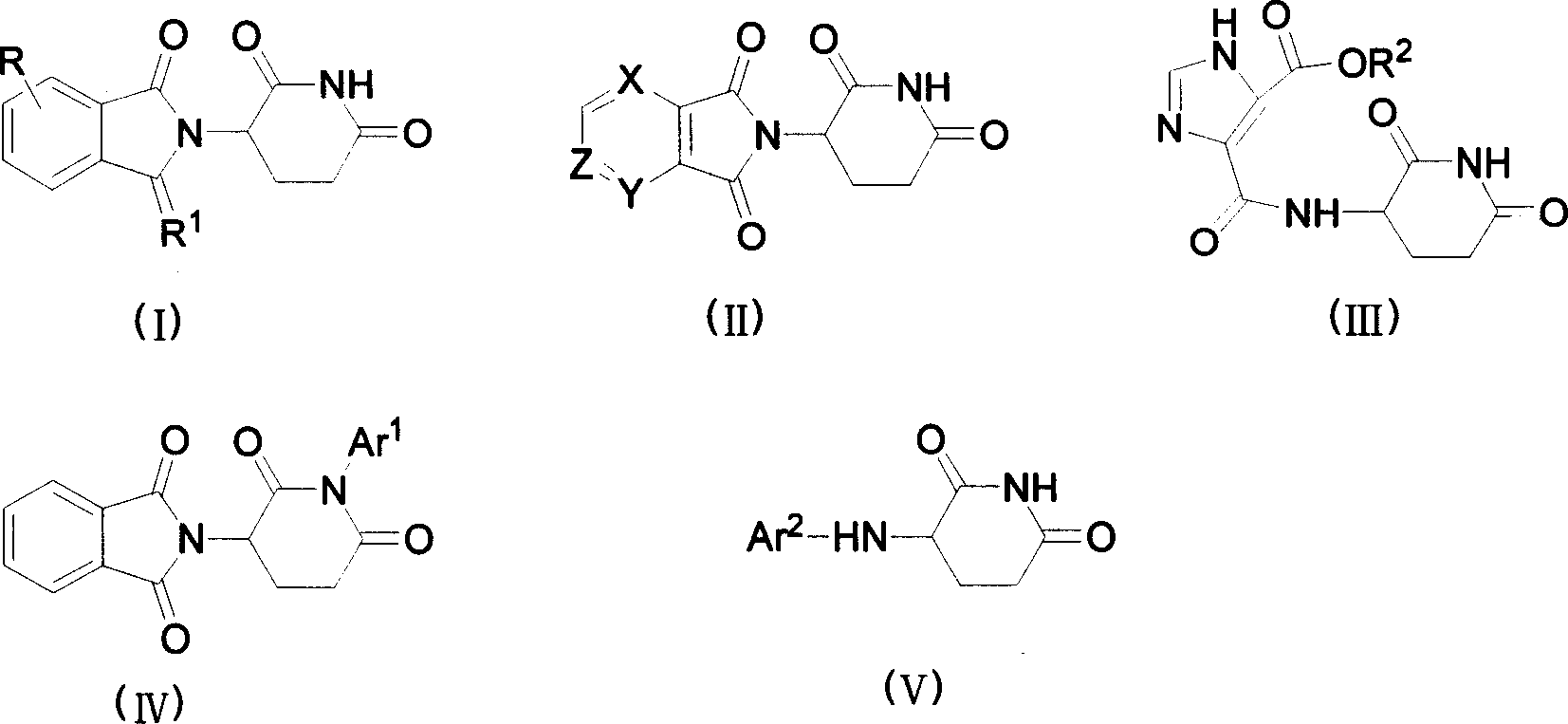
Analogue of thalidomide and preparation method
A technology of thalidomide and its analogues, which can be applied in drug combinations, bone diseases, non-central analgesics, etc., and can solve problems such as fatigue and constipation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add 4.0g of 3-nitrophthalic anhydride and 2.0g of glutamine into the reactor preheated to 140±10°C, heat and stir, and slowly raise the temperature to 180±10°C after 40 minutes of reaction. Connect the system to a vacuum pump, stop the reaction after 10 hours, add 24ml of 1,4-dioxane, heat and stir to make the system into a solution, distill out the dioxane under reduced pressure, add 20ml of methanol, reflux for 20 minutes, cool overnight, and a precipitate forms , filtered, and washed with methanol to obtain a pale white solid, which was dried in vacuo to obtain 1.46 g of thalidomide substituted with the 3-nitro group, with a yield of 35.2% and a melting point of 297-299°C. 1 HNMR (DMSO-d 6 ): б11.16 (s, 1H), 8.18-7.96 (m, 3H), 5.26-5.18 (dd, 1H), 2.96-2.87 (m, 1H), 2.68-2.56 (m, 2H), 2.15-2.08 (m, 1H); 13 CNMR (DMSO-d 6 ): δ: 172.7, 169.5, 165.2, 162.5, 144.4, 136.8, 133.0, 128.9, 127.3, 122.6, 49.5, 30.9, 21.8. Elemental analysis (%, C 13 h 9 N 3 o 6 Calcd): ...
Embodiment 2
[0043] In the autoclave, add the thalidomide 1.1g that substituted 3 nitro groups obtained in Example 1, 3.3ml of aqueous formaldehyde solution of 40%, methyl alcohol 100ml, 0.5g of palladium carbon of 10%, pass into hydrogen, make hydrogen The pressure was 30 atmospheres, the system was heated to 80°C, and the reaction was stopped after 40 hours. The system was filtered with filter aid, the filtrate was adsorbed by silica gel, the solvent was removed by rotary evaporation, and the 3 : EtOAc = 10: 1 is the eluent through a silica gel column to obtain a yellow solid, which is dried in vacuo to obtain 0.83 g of 3-position N, N-dimethyl-substituted thalidomide (7), with a yield of 82.5%, and a melting point of: 207-209°C. 1 HNMR (DMSO-d 6 ): б11.06(s, 1H), 7.65-7.61(dd, 1H), 7.28-7.22(m, 2H), 5.10-5.06(dd, 1H), 3.04(s, 6H), 2.89-2.85(m , 1H), 2.61-2.51(m, 2H), 2.06-2.00(m, 1H); 13 CNMR (DMSO-d 6 ): δ: 172.8, 170.0, 167.1, 166.3, 149.8, 135.1, 133.9, 122.5, 113.4, 112.9, 48.8...
Embodiment 3
[0045] 0.6g of the 3-position N, N-dimethyl-substituted thalidomide (7) obtained in Example 2 and 5g of zinc powder activated with hydrochloric acid reacted at 90°C for 40min in 50ml of glacial acetic acid and filtered while hot, 50ml Acetone washes three times, mother liquor adds silica gel adsorption, chloroform: ethyl acetate=4: 1 crosses chromatographic column, obtains and N, the analogue (8 )0.20g, yield 34.9%, melting point: 92-94°C. 1 HNMR (DMSO-d 6 ), δ: 10.96(s, 1H), 7.46-7.41(m, 1H), 7.00-6.97(d, 1H), 6.88-6.86(d, 1H), 5.08-5.02(dd, 1H), 4.38-4.18 (dd, 2H), 2.92(s, 6H), 3.01-2.85(m, 1H), 2.65-2.51(m, 1H), 2.40-2.36(m, 1H), 2.16-1.95(m, 1H). 13 CNMR (DMSO-d 6 ), δ: 173.3, 171.6, 168.1, 151.2, 145.6, 132.8, 120.1, 115.3, 114.5, 52.1, 47.0, 44.0, 31.7, 22.9. Elemental analysis (%, C 15 h 17 N 3 o 3 Calcd): C 62.549 (62.71), H 5.99 (5.96), N 14.80 (14.63).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com