PEGylated dendritic macromolecule drug carrier and preparation method thereof
A macromolecular and dendritic technology, which is applied in the field of PEGylated dendritic macromolecular drug carriers and its preparation, can solve the problems of limited application prospects, adding hydrophobic drugs, and affecting drug loading effects, and achieves a single molecular weight distribution and easy reaction , The effect of improving the efficiency of synthesis work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1 is G2.0 (NH 2 ) Synthesis of 18
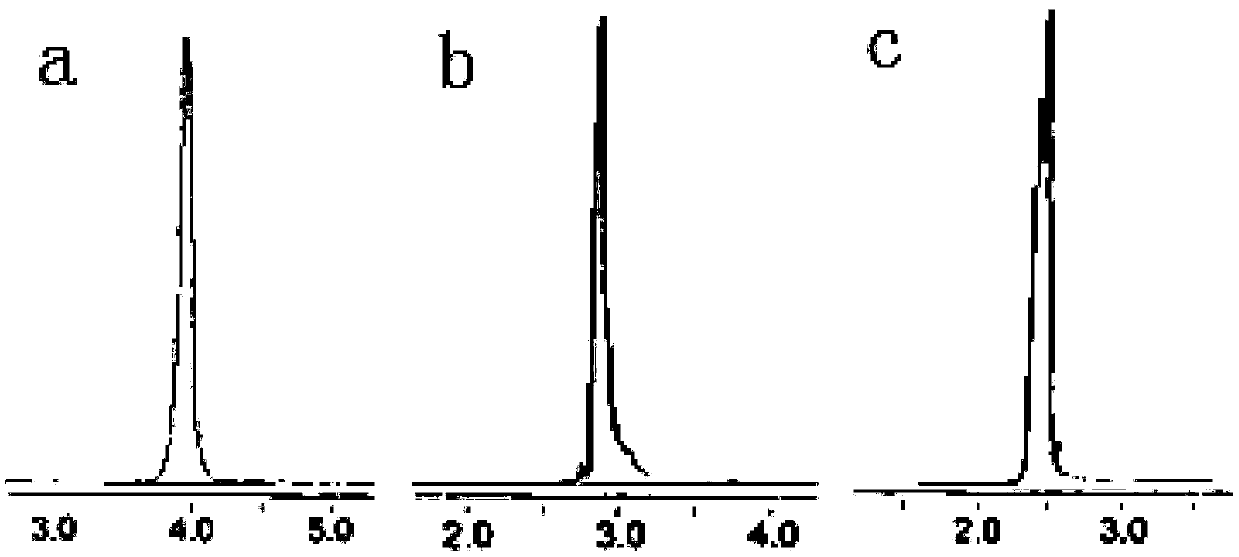
[0050] 1. Weigh 3.0g of TMPTA monomer, dissolve it in 2.4g of methanol, add 12g of ethylenediamine in an ice-water bath, stir and mix evenly in a 50mL three-necked bottle, raise the temperature to 45°C, and protect it under nitrogen The mixture was stirred and reacted for 18 hours, methanol and most of ethylenediamine were distilled off under reduced pressure, washed three times with 8 g, 6 g, and 6 g of ethyl acetate respectively, and vacuum-dried at 30°C to obtain G1.0 (NH 2 ) 3, its HPLC test result is as figure 1 as shown in a.
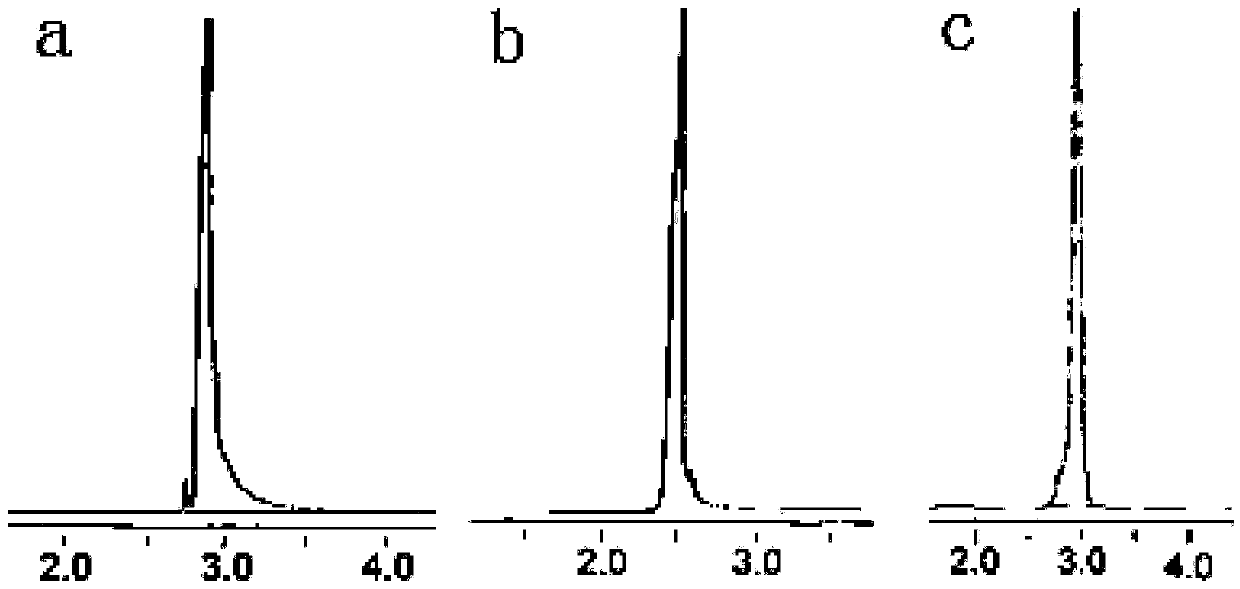
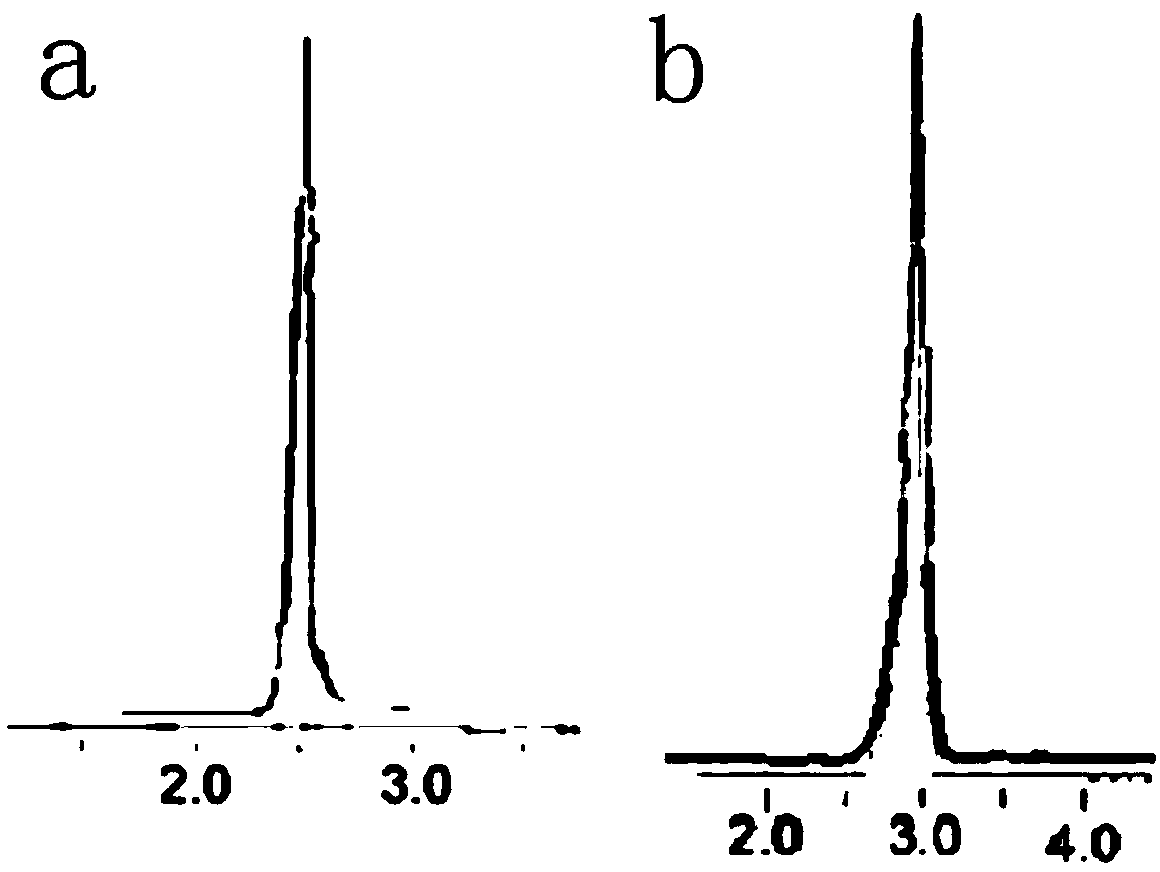
[0051] 2. Weigh G1.0 (NH 2 0.5 g of )3 dendrimers were dissolved in 4.0 g of methanol, and then 12.0 g of TMPTA was added, and stirred and reacted at 25° C. for 18 h. First, methanol and most of ethylenediamine were distilled off under reduced pressure, then washed three times with 8g, 6g, and 6g of ethyl acetate respectively, and dried in vacuum at 30°C to obtain G1.5 (acrylate) 18. The HP...
Embodiment 2
[0053] Example 2 The amino-terminated dendrimer with ethylenediamine as the core is G2.0 (NH 2 ) Synthesis of 18
[0054] Prepare G2.0 (NH 2 )18, the difference is:
[0055] In step 1, the mass ratio of TMPTA, ethylenediamine and organic solvent is 6:3:5, the reaction temperature is 60° C., and the reaction time is 10 h.
[0056] In step 2, G1.0(NH 2 ) 3. The mass ratio of TMPTA to the organic solvent is 0.1:2:0.5, the reaction temperature is 30°C, and the reaction time is 30h.
[0057] In step 3, the mass ratio of G1.5(acrylate)18, ethylenediamine and organic solvent is 0.6:10:1, the reaction temperature is 40° C., and the reaction time is 10 h.
Embodiment 3
[0058] Example 3 The amino-terminated dendrimer with ethylenediamine as the core is G2.0 (NH 2 ) Synthesis of 18
[0059] Prepare G2.0 (NH 2 )18, the difference is:
[0060] In step 1, the mass ratio of TMPTA, ethylenediamine and organic solvent is 2:10:1, the reaction temperature is 30°C, and the reaction time is 15h.
[0061] In step 2, G1.0(NH 2 ) 3. The mass ratio of TMPTA and organic solvent is 0.5:25:2, the reaction temperature is 40°C, and the reaction time is 45h.
[0062] In step 3, the mass ratio of G1.5(acrylate)18, ethylenediamine and organic solvent is 0.3:5:0.6, the reaction temperature is 30°C, and the reaction time is 15h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com