Process for preparing high purity breviscapine B raw material medicine
A technology for preparing scutellarin and its preparation technology, which is applied in the field of raw material medicine preparation technology, can solve the problems of improving the purity of scutellarin and eliminating side effects that have not been reported, and achieves easy industrial production, reduced toxicity, and simple process steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
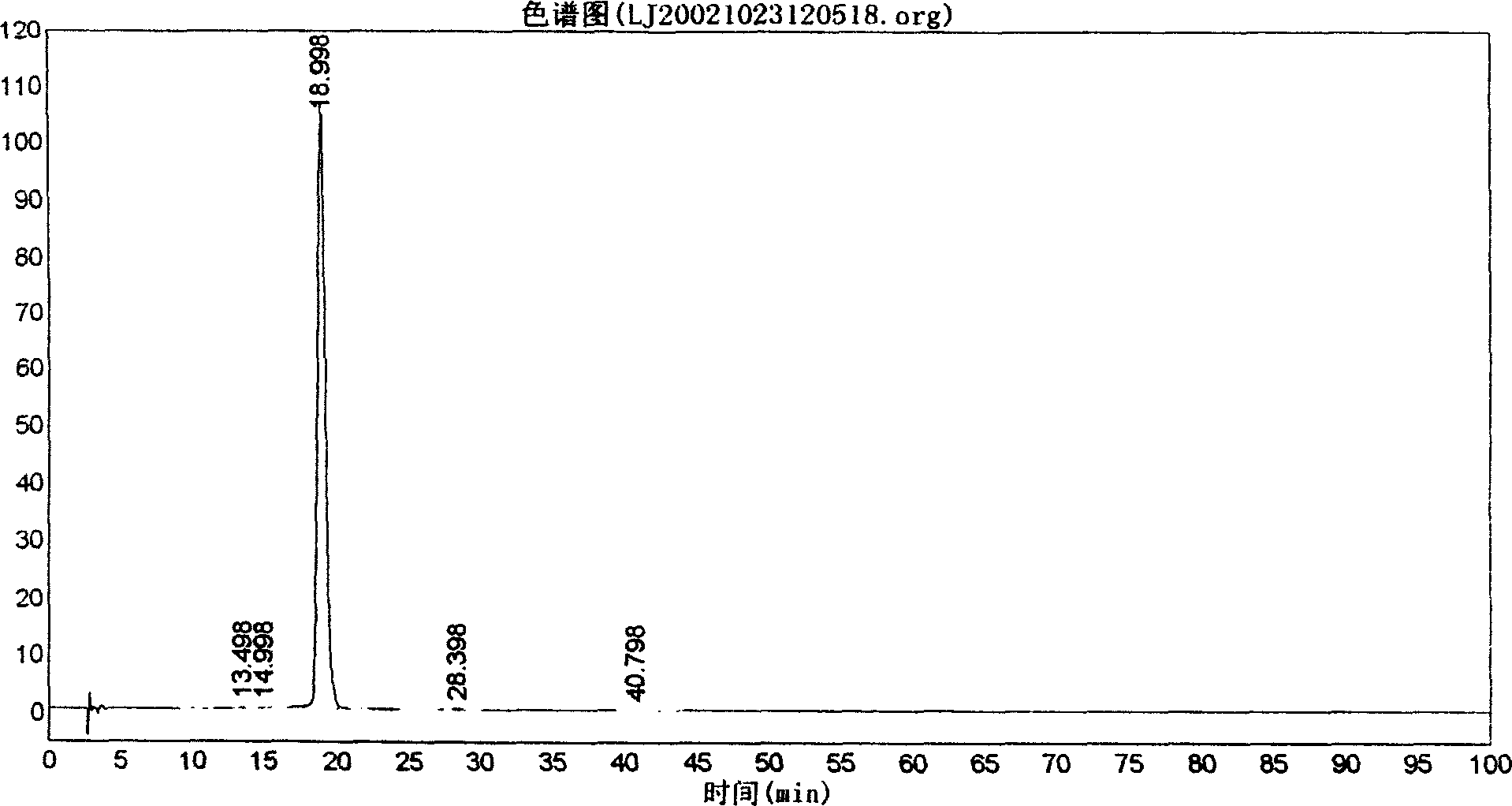
[0021] Weigh 1000g of commercially available breviscapine crude drug, add 5 times the volume of water, adjust the pH value to 7 with 20% disodium hydrogen phosphate test solution, make it completely dissolved, filter, add 8 times of acetone to the filtrate to precipitate, stir while adding, Make the precipitation complete, let stand for 10 hours, filter, add acetone to wash three times, then move the precipitate to another container, add 5 times the amount of 30% acetone, stir well, add 20% hydrochloric acid to adjust the pH to 1-2 Set it aside for 10 hours, filter with suction, wash with water until neutral, wash once with ethanol, and dry to obtain refined scutellarin. The content of scutellarin by HPLC analysis is 99.43%, as shown in the attached figure.
[0022] Detection method: chromatographic conditions and system suitability test (using octadecyl bonded silica gel as filler: methanol-0.1% phosphoric acid solution (40:60) as mobile phase; detection wavelength is 335nm. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com