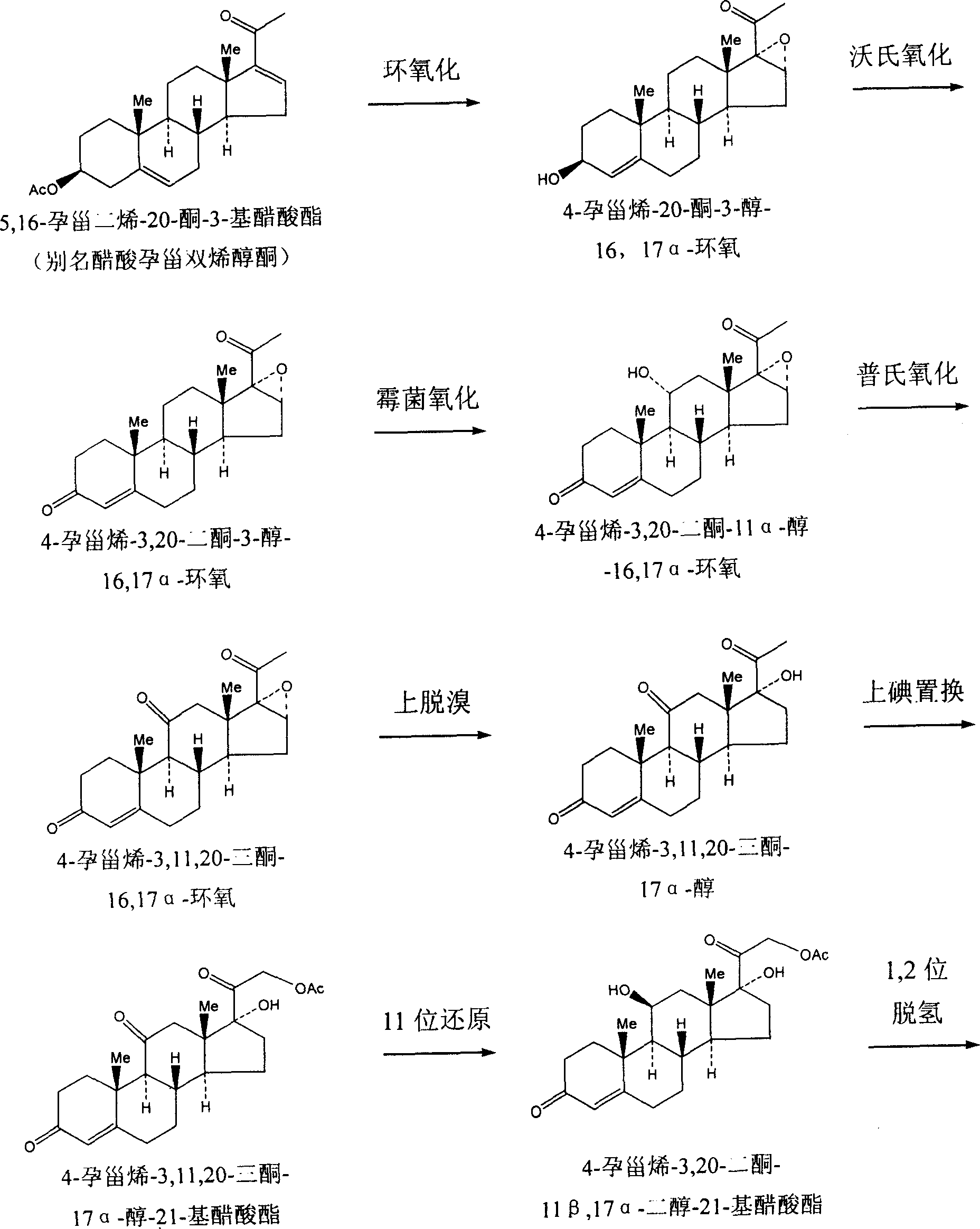
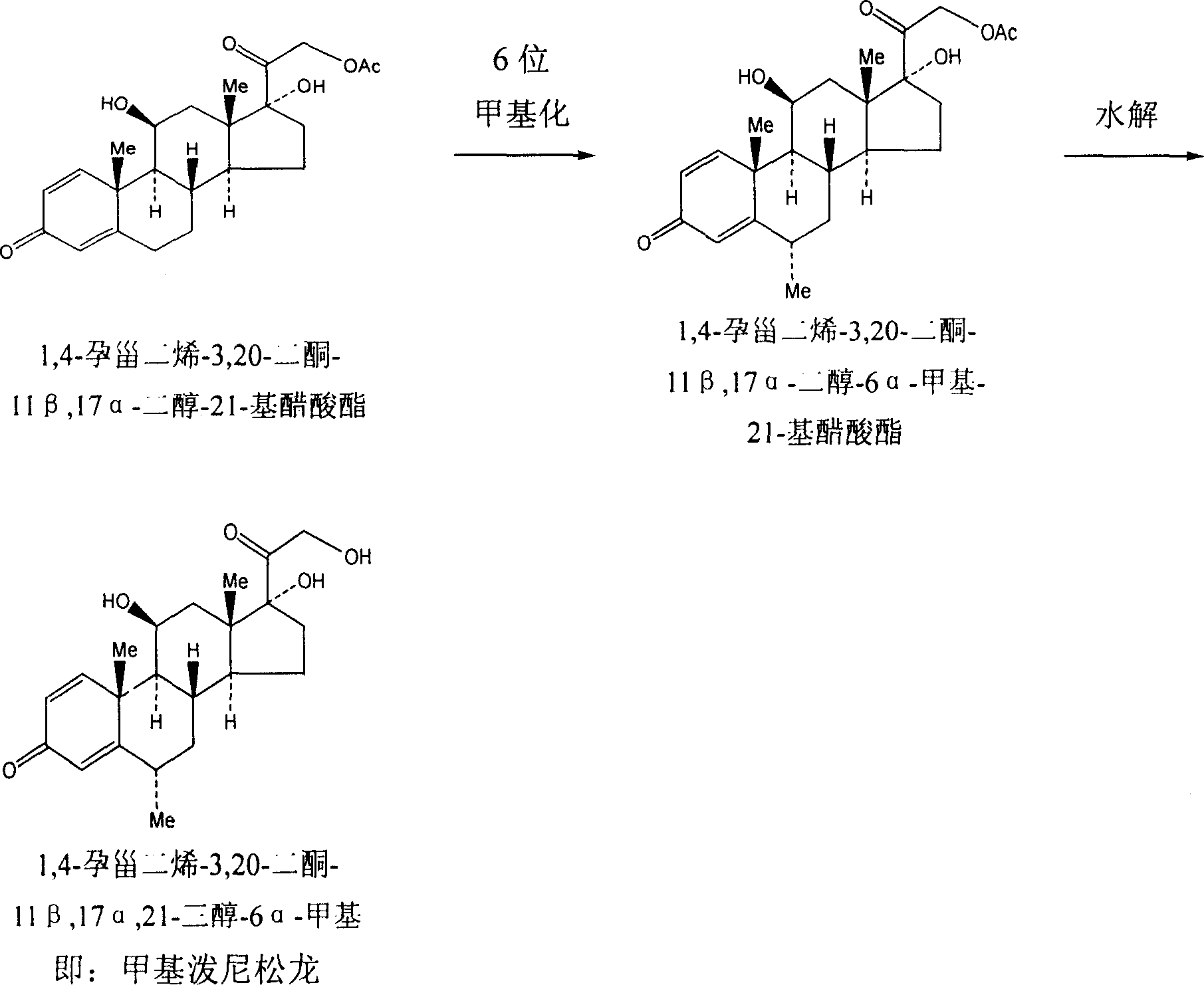
Methylprednisolone chemical synthesis method
A technology of methylprednisolone and chemical synthesis, applied in organic chemistry, steroids, etc., can solve the problems of many reaction by-products, incomplete methylation transposition, large synthesis reaction side reactions, etc. The effects of improved efficiency and quality, easy industrialization, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Pregnene dienolone acetate starting material 30 kg, 4-pregnene-3,11,20- 24 kilograms of triketone-17α-alcohol-21--base acetate; Add 160 kilograms of dichloromethane, 50 kilograms of triethyl formate, 30 kilograms of ethylene glycol, 1.5 kilograms of p-toluenesulfonic acid, and carry out at room temperature for 3 , the 20-position ketone group protection reaction; then add 15 kg of potassium borohydride, 300 kg of pyridine, add 1-2 times of water, and carry out the 11-position reduction reaction for 15-30 hours; add 19.5 kg of benzoquinone and p-toluenesulfonic acid to the reaction product 1.7 kilograms react under 350 kilograms of methyl alcohol: chloroform=6: 5 mixed solvents; Add 80 kilograms of dimethylformamides and make solvent in the reaction product, 4.5 kilograms of palladium charcoals are made catalysts, and 20 kilograms of cyclohexenes are made reaction aids, 100 The product obtained after reacting at ℃ for 2-3 hours was hydrolyzed to obtain 12.5 kg of methylp...
Embodiment 2
[0020] Pregnene dienolone acetate starting material 30 kg, 4-pregnene-3,11,20- 24 kilograms of triketone-17α-alcohol-21--base acetate; Add 160 kilograms of dichloromethane, 25 kilograms of triethyl formate, 30 kilograms of ethylene glycol, 1.5 kilograms of p-toluenesulfonic acid, and carry out at room temperature for 3 , the 20-position ketone group protection reaction; then add 15 kg of potassium borohydride, 300 kg of pyridine, add 1-2 times of water, and carry out the 11-position reduction reaction for 15-30 hours; add 19.5 kg of benzoquinone and p-toluenesulfonic acid to the reaction product 1.7 kilograms react under 350 kilograms of methyl alcohol: chloroform=6: 5 mixed solvents; Add 80 kilograms of dimethylformamides and make solvent in the reaction product, 4.5 kilograms of palladium charcoals are made catalysts, and 20 kilograms of cyclohexenes are made reaction aids, 100 The product obtained after reacting at ℃ for 2-3 hours was hydrolyzed to obtain 11.3 kg of methylp...
Embodiment 3
[0022] Pregnene dienolone acetate starting material 30 kg, 4-pregnene-3,11,20- 24 kilograms of triketone-17α-alcohol-21--base acetate; Add 160 kilograms of dichloromethane, 30 kilograms of triethyl formate, 30 kilograms of ethylene glycol, 1.5 kilograms of p-toluenesulfonic acid, and carry out at room temperature for 3 , the 20-position ketone group protection reaction; then add 15 kg of potassium borohydride, 300 kg of pyridine, add 1-2 times of water, and carry out the 11-position reduction reaction for 15-30 hours; add 19.5 kg of benzoquinone and p-toluenesulfonic acid to the reaction product 1.7 kilograms react under 350 kilograms of methyl alcohol: chloroform=6: 5 mixed solvents; Add 80 kilograms of dimethylformamides and make solvent in the reaction product, 4.5 kilograms of palladium charcoals are made catalysts, and 20 kilograms of cyclohexenes are made reaction aids, 100 The product obtained after reacting at ℃ for 2-3 hours was hydrolyzed to obtain 11.8 kg of methylp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com