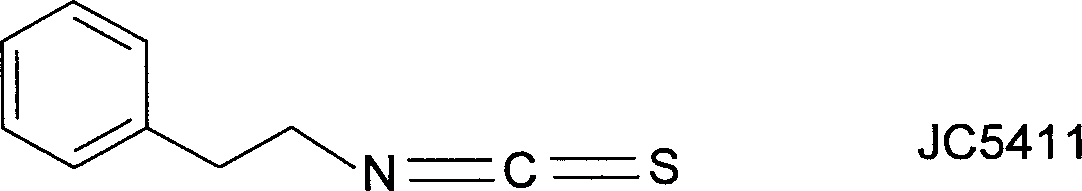Use of natural or artificial isorhodanate analog compound JC-5411 in preparing transcription factor SP1 inhibitor
A technology of JC-5411, ester compounds, applied in the field of medical applications, can solve problems such as uncertainty of curative effect, and achieve the effects of low production cost, inhibition of abnormal proliferation, and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, the synthesis of JC-5411:
[0059] Instruments and Reagents
[0060] 1 H-NMR spectrum is measured with BruckerAV-300 nuclear magnetic resonance instrument, internal standard TMS, solvent is CDCl 3 ; MS was determined with a Nicolet FTMS-2000 mass spectrometer; elemental analysis was determined with an Elementar Vario EL III instrument.
[0061] Thin-plate chromatography (TLC) was self-made with silica gel GF254 (produced by Qingdao Ocean Chemical Factory); all reagents were commercially available chemically pure or analytically pure products, and were used directly without treatment.
[0062] Preparation and Inspection of JC-5411 (Phenethyl Isothiocyanate)
[0063] Into a 50 mL round bottom flask was added 15 mL of CH 2 Cl 2 and 3 mL (40 mmol) of thiophosgene, stirred, cooled to 0 ° C, and slowly added dropwise an equivalent amount of triethylamine (4.04 g, 40 mmol) and phenethylamine (4.85 g, 40 mmol) with a constant pressure dropping funnel. Mixe...
Embodiment 2
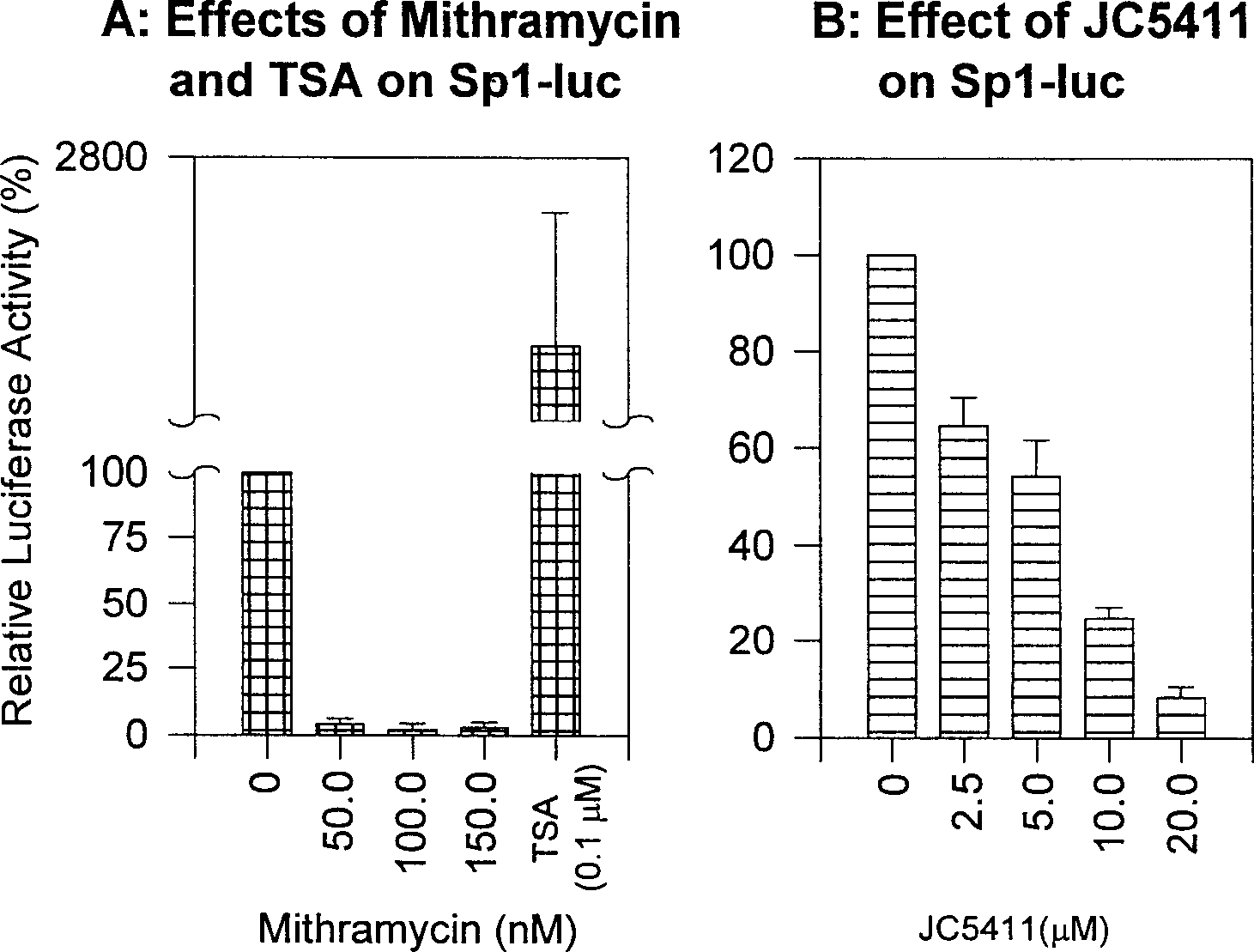
[0068] Example 2, JC-5411 inhibits the activity of transcription factor SP1:
[0069] Materials and methods:
[0070] Reagent: Take the JC-5411 product synthesized above to make a solution in DMSO. Sp1-luc and mtSp1-luc are composed of the luciferase gene and the corresponding mutant gene whose promoter contains 3 repeats of the SP1 regulatory sequence, and are used to measure the activity of the SP1 transcription factor (40) . Effectene Transfection Reagent and Luciferase Assay Kit were purchased from Qiagen (Valencia, CA) and Promega (Madison, WI), respectively. Western Blot ELC Kit was purchased from Amersham Biosciences (Buckingamshire, England). Polyacrylamide gel reagents for protein electrophoresis, SDS, electrophoresis buffer, and protein electrotransfer buffer were purchased from Bio-Rad Life Sciences, USA. Unless otherwise specified, other chemical reagents used in the experiment were purchased from Sigma Chemical Reagent Company in the United States.
[0071] C...
Embodiment 3
[0078] Example 3, Inhibition of JC-5411 on Androgen Receptor Transcription and Expression
[0079] Materials and Methods
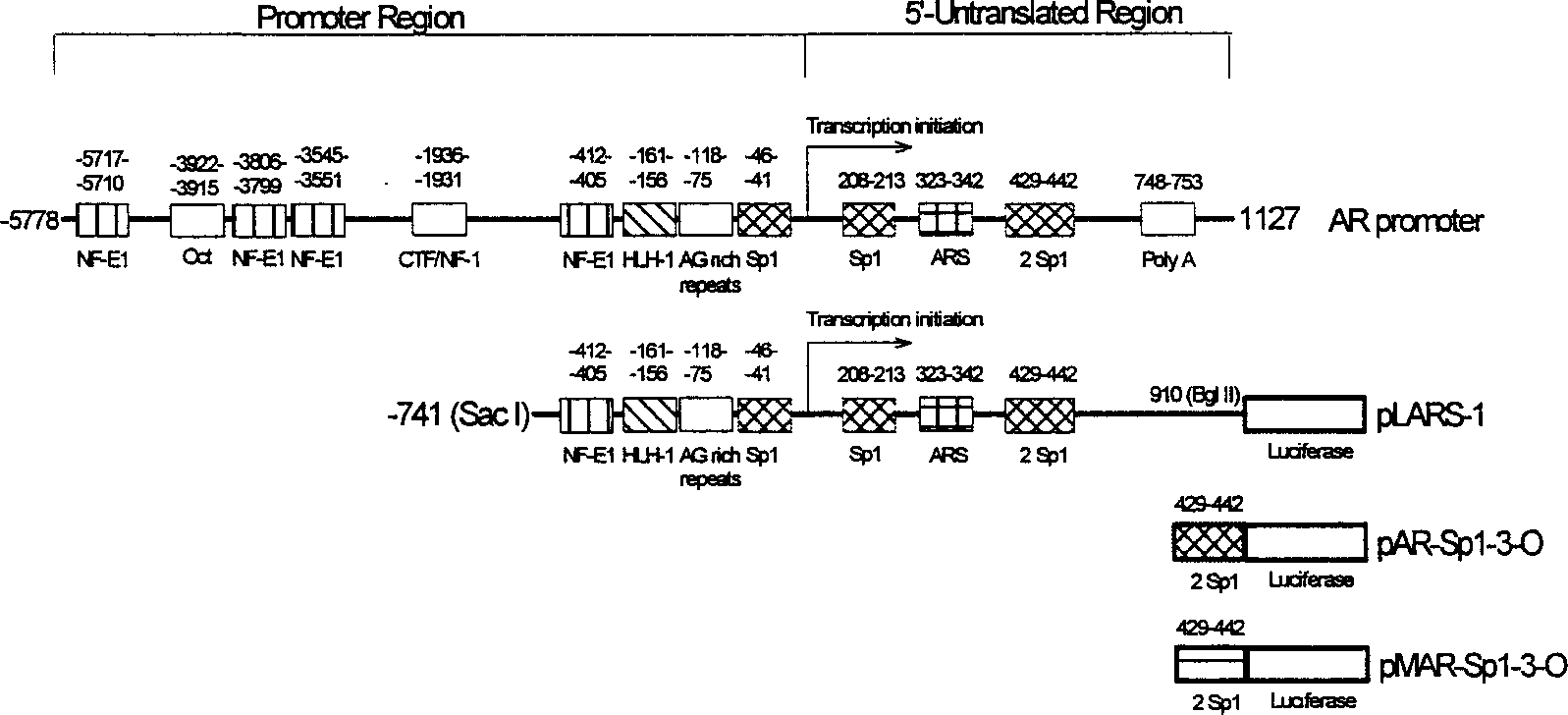
[0080] Reagent: JC-5411, same as Example 2. AR transfection gene see reference (5) . SP1-specific androgen receptor (AR)-luciferase transfection gene (pAR-Sp1-3-O, see figure 1 ), by inserting 3 repeats of the SP1 sequence (located at +429~+442 of the AR promoter) or the corresponding mutant sequence in the luciferase basic gene pGL3 (no promoter), the sequence of which was analyzed by DNA sequence to ensure Exactly. Effectene Transfection Reagent and Luciferase Assay Kit were purchased from Qiagen (Valencia, CA) and Promega (Madison, WI), respectively. AR, PSA and β-actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Western Blot ELC Kit was purchased from Amersham Biosciences (Buckingamshire, England). Polyacrylamide gel reagents for protein electrophoresis, SDS, electrophoresis buffer, and protein electrotransfer buffer we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com