Orally taken nanometer protein polypetide composition particle and its prepn
A technology of protein peptides and complexes, which is applied in the field of medicine, can solve the problems of phospholipid bilayer membrane instability, protein instability, and short half-life, and achieve the effects of improving oral bioavailability, ensuring biological activity, and mild preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Embodiment 1: Preparation of insulin-phospholipid complex by vacuum evaporation method
[0011] Take 50mg of porcine insulin powder and 1000mg of soybean lecithin for injection and dissolve in 5ml of acidic ethanol solution, and stir at 400r / min until a clear solution is formed. The solution was evaporated under reduced pressure at 35° C. for 2 hours, and then dried again with nitrogen gas for 15 minutes to obtain a golden-yellow phospholipid film of composite insulin.
Embodiment 2
[0012] Embodiment 2: Preparation of insulin-phospholipid complex by freeze-drying method
[0013] Take 30 mg of insulin and 800 mg of soybean lecithin for injection and dissolve them in 5 ml of dimethyl sulfoxide, and stir at 400 r / min until a clear solution is formed. This solution was lyophilized for 12 hours to obtain a dry white loose powder.
Embodiment 3
[0014] Example 3: Preparation of polylactic acid co-precipitated lipid complex nanoparticles containing insulin
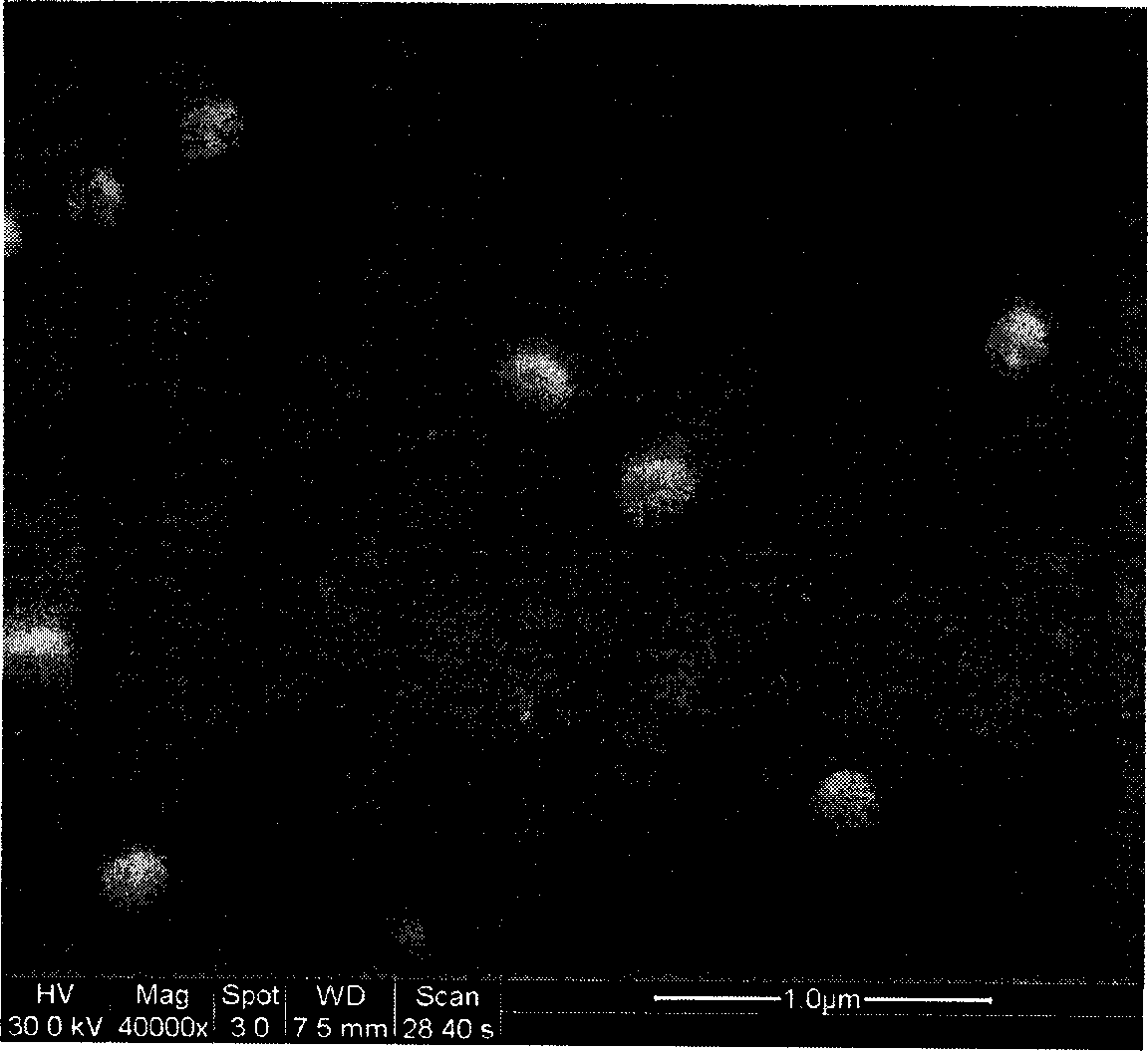
[0015] Dissolve 500 mg of DL-polylactic acid with a molecular weight of 10,000 in 5 ml of dichloromethane-acetone mixed solvent (the ratio of the two is 1:5), and stir evenly. Add 50 mg of the insulin-containing phospholipid membrane prepared in Example 1 into the polymer solution, and stir until clear. The above solution was poured into an aqueous solution containing 2% polyvinyl alcohol, stirred at room temperature at 400 r / min for 6 h, and after the solvent was evaporated, a blue opalescent nanosuspension was obtained. SEM photo see figure 1 . The measured encapsulation efficiency was 92%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com