Method for synthesizing di-ferrocene phosphine diimine structure connected with aliphatic series and benzene ring
A bis-ferrocene phosphine diimine and synthesis method technology, applied in chemical instruments and methods, metallocenes, organic chemistry, etc. Problems such as the application of iron derivatives, to achieve the effects of good yield and enantioselectivity, good application prospects, high catalytic activity and chiral induction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
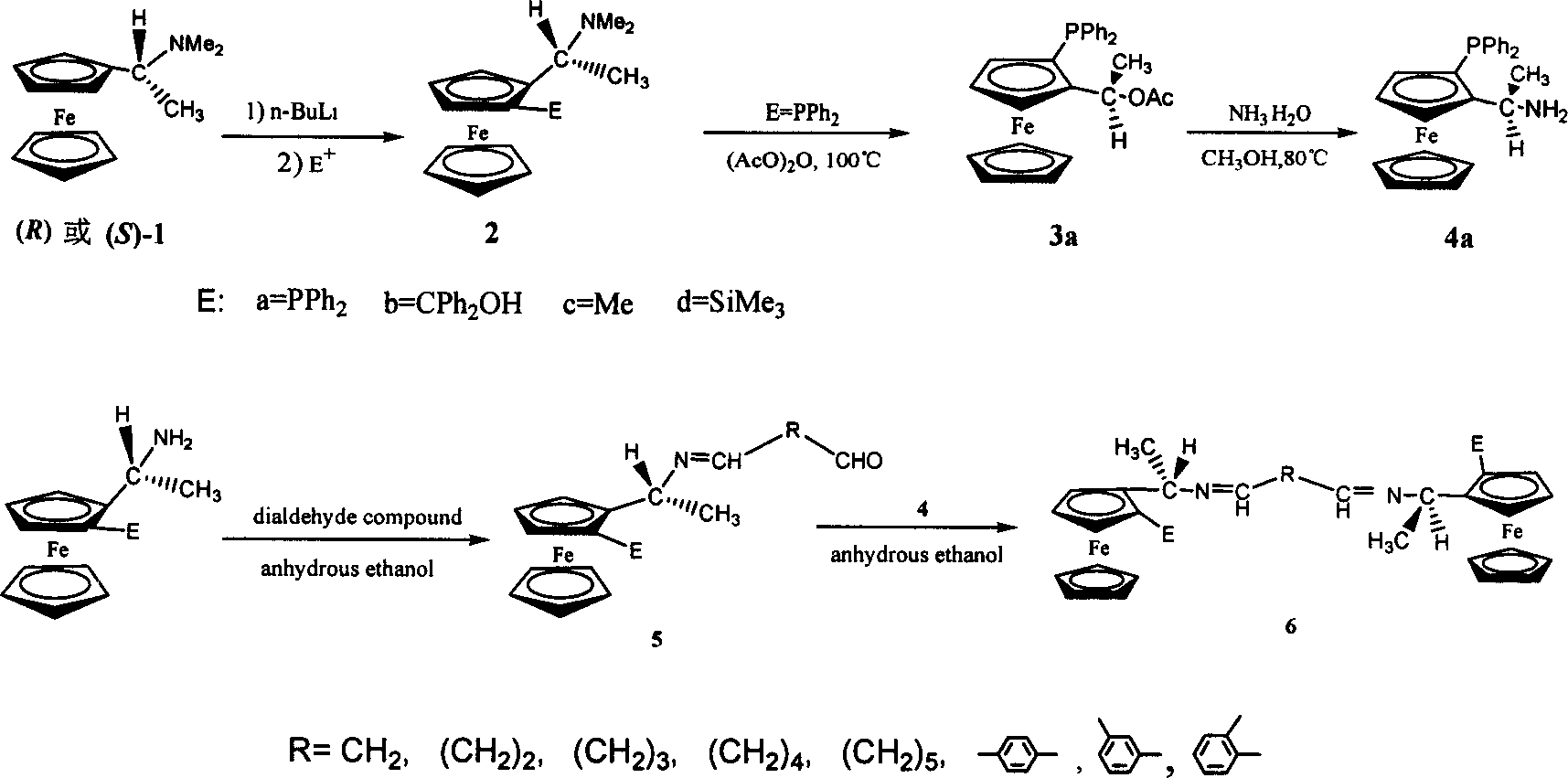
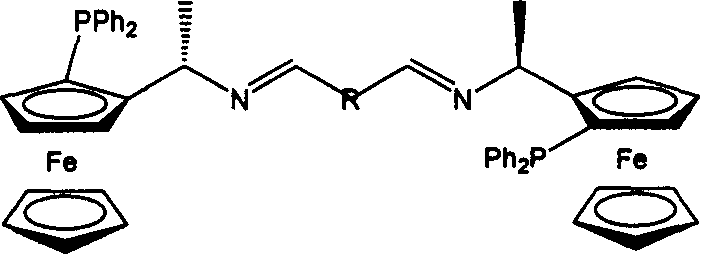
[0023] (R)-N, N-dimethyl-1-ferrocenylethylamine (the resolution of synthesis and racemate is carried out according to the method reported by D.Marquarding et al.) was dissolved in anhydrous ether, and used Lithiation of n-butyllithium followed by quenching with diphenylphosphine chloride gave [(R,S p )-2]. will [R, S p )-2] was dissolved in anhydrous acetic anhydride, reacted at 100°C for 2h, and crystal 3 was precipitated. Crystal 3 was dissolved in methanol, excess ammonia water was added, and reacted at 80° C. for 7 h to obtain ferrocene primary amine 4 . Ferrocenylphosphinoamine compound 4 (868 mg, 2.1 mmol) and glutaraldehyde (395 mg, 4.2 mmol) were dissolved in absolute ethanol, molecular sieves (5 g) were added to reflux for 4 hours, and the ethanol was distilled off under reduced pressure to obtain a red solid. Wash with hot water to remove unreacted excess glutaraldehyde (10ml×2), dry to obtain crude product 5, dissolve 5 in ethanol and add ferrocenylphosphine amin...
Embodiment 2
[0026] Condition is the same as embodiment 1, only terephthalaldehyde is changed into m-phthalaldehyde.
Embodiment 3
[0028] Condition is the same as embodiment 1, only terephthalaldehyde is changed into o-phthalaldehyde.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com