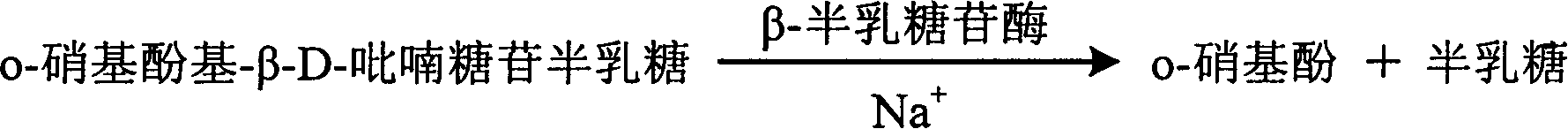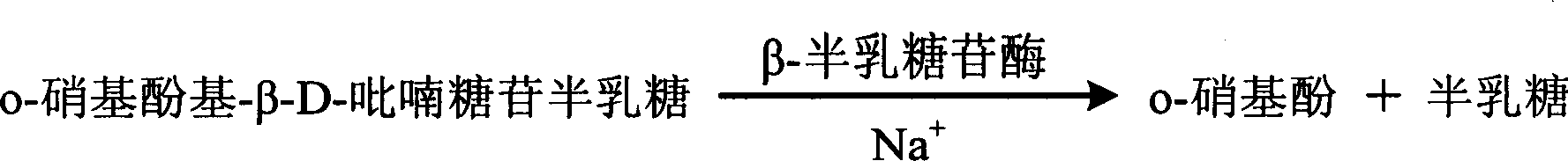Enzymatic method for determining sodium ion content and sodium ion diagnosis kit
A diagnostic kit and sodium ion technology are applied in the field of medical testing and determination, which can solve the problems that a biochemical analyzer cannot measure the content of sodium ions and potassium ions at the same time, cannot be popularized and applied, and are complicated to operate, so as to achieve low testing cost and easy popularization. The effect of simple application and method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 (single dose)
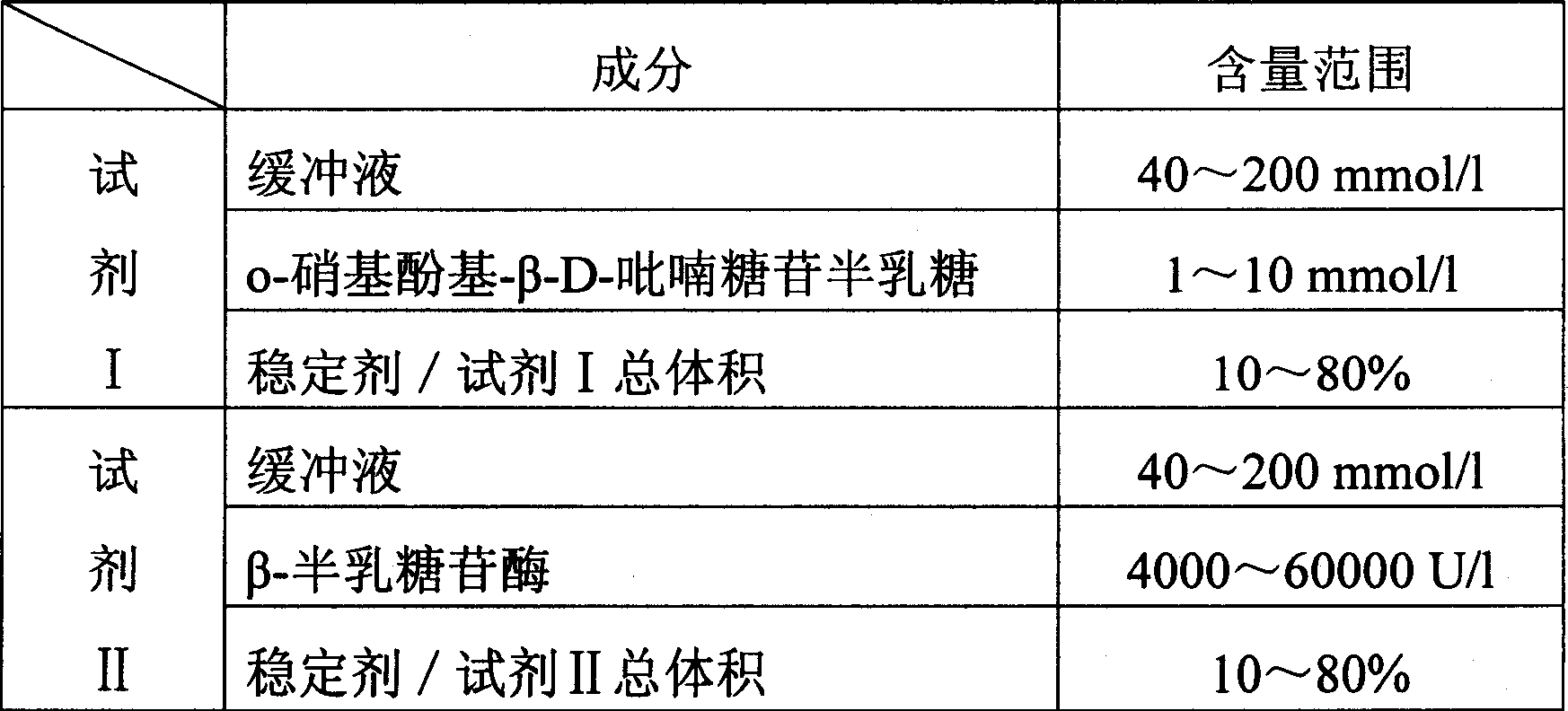
[0039] Prepare a sodium ion diagnostic kit with the following ingredients and amounts:
[0040] Diglycine buffer 80mmol / l,
[0041] o-Nitrophenol-β-D-galactopyranoside 2mmol / l,
[0042] β-Galactosidase 10000U / l,
[0043] Ethylene glycol 50% (total reagent volume).
[0044] Set on the automatic biochemical analyzer (Hitachi-7080): temperature 37°C, reaction time 10 minutes, test main wavelength 405nm, test sub-wavelength above 505nm, the volume ratio of the tested sodium ion sample to the reagent is 1:25, The reaction direction is a positive reaction (the absorbance increases, the same below).
[0045] Add the sample and the prepared single agent, and the two are automatically mixed inside the analyzer to detect and record the rise of the absorbance at 405 nm. According to the rate method, end point method and other methods, the content of sodium ions in the sample was measured against the corresponding standard curve. ...
Embodiment 2
[0046] Embodiment 2 (one of the two-dose examples)
[0047] Prepare a sodium ion diagnostic kit with the following ingredients and amounts:
[0048] Reagent I——
[0049] Imidazole-hydrochloric acid buffer 100mmol / l,
[0050] o-Nitrophenol-β-D-galactopyranoside 5mmol / l,
[0051] Glycerol 20mmol / l;
[0052] Reagent II——
[0053] Imidazole-hydrochloric acid buffer 100mmol / l,
[0054] β-Galactosidase 20000U / l,
[0055] Ethylene glycol 50% (total volume of Reagent II).
[0056] Set on the automatic biochemical analyzer (Hitachi-7080): the temperature is 30°C, the reaction time is 15 minutes, the test main wavelength is 405nm, and the test sub-wavelength is above 505nm. The total volume of the tested sodium ion sample and reagent I and reagent II is The ratio is 1:25, the ratio of the amount of reagent I to reagent II is 4:1, and the reaction direction is positive reaction.
[0057] Add the sample and reagent I first, and add reagent II after 5 ...
Embodiment 3
[0058] Embodiment 3 (two-agent example)
[0059] Prepare a sodium ion diagnostic kit with the following ingredients and amounts:
[0060] Reagent I——
[0061] Tris-HCl buffer 120mmol / l,
[0062] β-Galactosidase 30000U / l,
[0063] Glycerol 50% (total volume of reagent I);
[0064] Reagent II——
[0065] Tris-HCl buffer 120mmol / l,
[0066] o-Nitrophenol-β-D-galactopyranoside 8mmol / l,
[0067] Propylene glycol 50% (total volume of Reagent II).
[0068] Set on an automatic biochemical analyzer (Hitachi-7080): temperature 37°C, reaction time 10 minutes, test main wavelength 405nm, test sub-wavelength above 505nm, the total volume of the tested sodium ion sample and reagent I and reagent II The ratio is 1:25, the ratio of the amount of reagent I to reagent II is 4:1, and the reaction direction is positive reaction.
[0069] Add the sample and reagent I first, and add reagent II after 5 minutes. The three are automatically mixed inside the analyze...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com