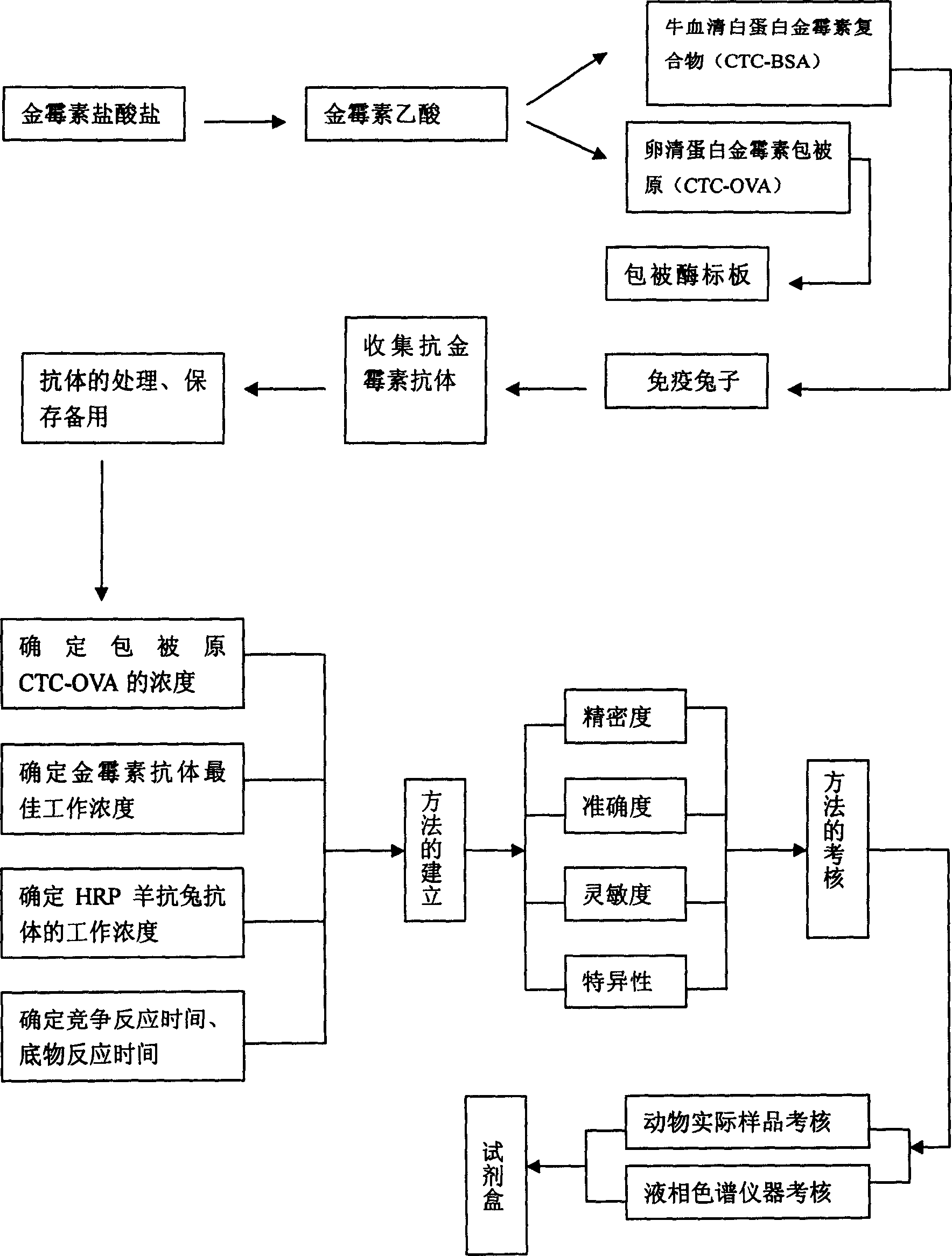
Enzyme-linked immune assay kit adapted to biomycin residue analysis and application
A detection kit and residue analysis technology, applied in analytical materials, measuring devices, instruments, etc., can solve problems such as unfavorable work efficiency, increase operation steps, increase detection cost, etc., to shorten detection time, reduce operation steps, reduce The effect of testing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 prepares aureomycin acetic acid, artificial immunogen, coating antigen, anti-aureomycin antibody
[0038] 1.1 Preparation of aureomycin acetic acid
[0039] Take by weighing 300mg aureomycin hydrochloride (can replace with aureomycin, aureomycin alkali in another embodiment, its usage amount is identical with aureomycin hydrochloride), dissolve with the phosphate buffer saline solution of 25mlpH10, add 58.5mg chlorine Sodium acetate, fully react at room temperature for 10 hours, filter, adjust the pH value to 4.0 with dilute hydrochloric acid, let stand for 2 hours, and dry at 50°C after suction filtration to obtain aureomycin acetic acid.
[0040] 1.2 Preparation of artificial immunogen
[0041] Dissolve 118.8 mg of aureomycin acetic acid with 2 ml of N, N-dimethylformamide (DMF), add N, N dicyclohexyl carboximide (DCC) 55 mg, N-hydroxysuccinimide (NHS) while stirring 28.8 mg, stirred at 4°C for more than 10 hours, centrifuged at 4°C (10000r / min), added ...
Embodiment 2
[0058] Embodiment 2 establishes aureomycin ELISA indirect competitive detection method (ELISA)
[0059] 2.1 Determination of the coating concentration of the original coating and the working concentration (dilution factor) of the aureomycin antibody
[0060] Determine the three reference points of high, middle and low by the square matrix titration test: the point with the absorbance value of about 1.0 and the difference between the left and right adjacent absorbance values is the largest as the reference point. Operation steps: Coat the first row of the 96-well ELISA plate with 8 μg / kg of coating source, and the second to seventh rows are coated with 4, 2, 1, 0.5, 0.25, 0.125 μg / kg of coating Original. Overnight at 4°C, block with 1% ovalbumin at 37°C for 1 hour, wash twice, pat dry, add 100 μl to the first column to the seventh column of the microtiter plate, and the dilution factor is 1000, 2000, 4000, 8000, 16000, 32000, 64000 chlortetracycline antibody, incubate at 37...
Embodiment 3
[0090] Embodiment 3 The accuracy and precision test of the present invention
[0091] 3.1 Now take pig muscle as an example to illustrate the processing method and results of meat tissue samples (the same test can use animal muscle tissue such as chicken or duck or cattle or sheep).
[0092]Add chlortetracycline standard substance with a concentration of 100 μg / kg into the porcine muscle tissue to be tested, and another porcine muscle tissue without chlortetracycline was used as a blank as a control, and each concentration was repeated 5 times. Operation steps: Weigh 5 grams of homogenized porcine muscle tissue for each sample, prepare test samples and blank samples according to the aforementioned method and dosage, then add 5 ml of pH4. Centrifuge at 4000 rpm, take the supernatant, add 0.3ml of 20% trichloroacetic acid, shake well and then centrifuge at 4°C, 8000 rpm, take the supernatant, adjust the pH value to 7.4 after 10-fold dilution (add 1N NaOH About 100 microliters),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com