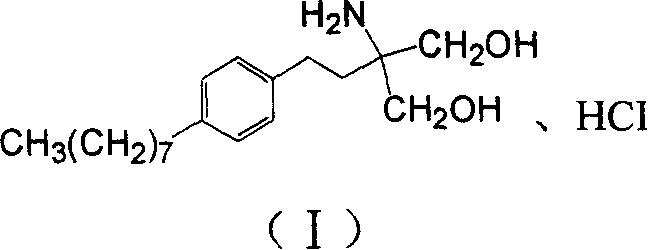
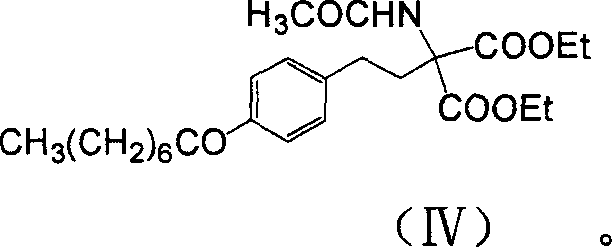
Method for preparing 2-P-octyl-phenenl-2-amino-propanediol hydrochloride
A technology of aminopropylene glycol hydrochloride and diethyl acetamidomalonate, which is applied in the field of medicinal immunosuppressants, can solve the problems of low total yield, high cost, and long reaction steps, and achieve high total yield, The effect of low production cost and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
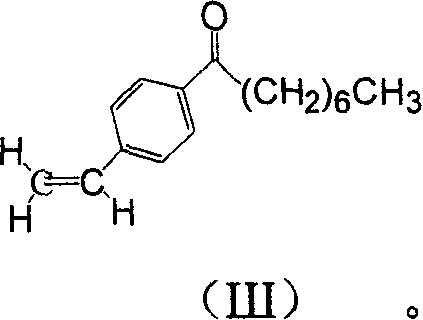
[0023] One, the preparation of compound p-octanoylstyrene (III):
[0024] At 0°C, add octanoyl chloride to a suspension of dichloromethane (200ml) in aluminum trichloride (32g, 0.24mol), stir at 0-5°C for 0.5h after the addition, add dropwise (II) (20g, 0.19mol), the ice-salt bath was removed after the dropwise addition, stirred at room temperature for 3h, and the end point was detected by TLC. The mixture was poured into ice water, and the organic layer was separated. The aqueous layer was extracted with dichloromethane (20ml×3). Wash with brine, dry the organic layer with anhydrous sodium sulfate, filter, evaporate the solvent, and recrystallize from a mixed solvent of petroleum ether and ethyl acetate (3:1) to obtain 39.35 g of p-octanoylstyrene (III) as colorless crystals , melting point 62-64°C, yield 90%.
[0025] 1 H NMR (CDCl 3 )δ: (d, J = 9.6Hz, 2H), 7.47 (d, 9.6Hz, 2H), 4.74 (dd, J = 13.2, 21.0Hz, 1H), 5.86 (d, J = 21.0Hz, 1H), 5.37(d, J=13.2Hz, 1H), 2.93(t, J=9....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com