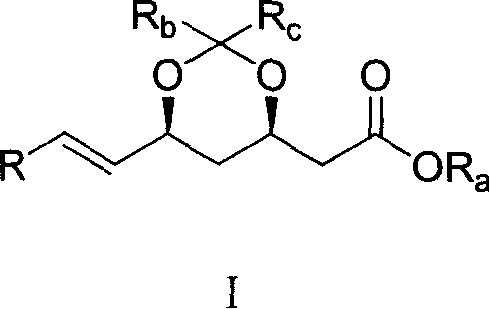
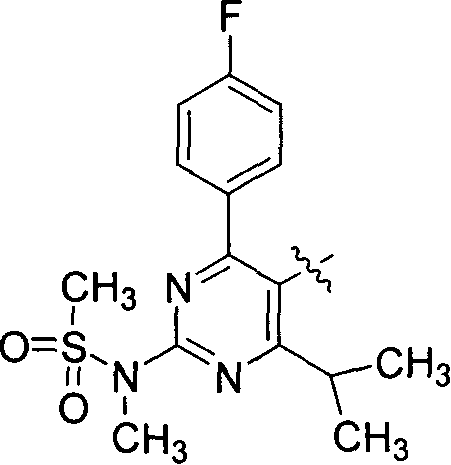
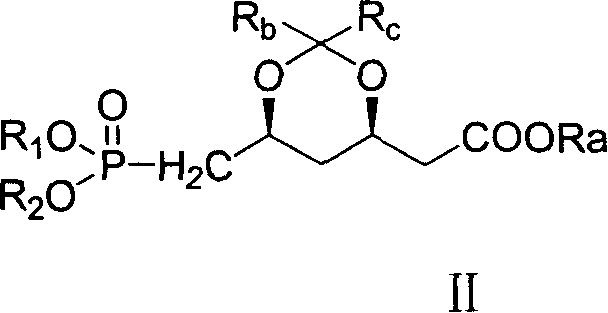
Novel method for preparing dihydroxy acid HMG CoA reductase inhibitor
A hydroxycarboxylate, alkyl technology, applied in the field of preparing dihydroxyacid HMG-CoA reductase inhibitors, can solve problems such as difficult operation, complex structure, difficult synthesis, etc., and achieves less environmental pollution, high yield, easy effect of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Preparation of compound (B) 4-(4-fluorobenzene)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)-5-pyrimidinecarbaldehyde:
[0058]Add 28.0 g of pyridinium chlorochromate (PCC) and 24.6 g of anhydrous sodium acetate to 300 ml of dry dichloromethane, stir, and add compound (A) [4-(4-fluorobenzene)-6-iso Propyl-2-(N-methyl-N-methylsulfonylamino)pyrimidin-5-yl]methanol 35.3g and 200ml dichloromethane prepared solution, stirred for 1h, filtered, filtrate 1N hydrochloric acid 500ml, 1N NaOH 500ml, Wash with 500 ml of saturated brine, dry over anhydrous magnesium sulfate, remove dichloromethane under reduced pressure, and perform column chromatography on the residue with dichloromethane as the eluent to obtain 29.1 g of the crystalline target product (yield: 82.9%).
Embodiment 2
[0059] Embodiment 2: the preparation of compound (D):
[0060] Add 13.0 g of trimethyl phosphite to 200 ml of toluene, raise the temperature to reflux, add compound (C) 2-[(4R,6S)-6-bromomethyl-2,2-dimethyl-1,3-dioxo -4-yl] tert-butyl acetate 32.3g and toluene 200ml prepared solution, reflux reaction for 3h, remove toluene under reduced pressure, add 500ml of water, extract with 200ml of chloroform×3, dry over anhydrous magnesium sulfate, remove chloroform under reduced pressure, The residue was subjected to column chromatography with ethyl acetate as the eluent to obtain 31.7 g of the target product (yield: 90.1%).
Embodiment 3
[0061] Embodiment 3: the preparation of compound (E)
[0062] Add 15.3g of LiCl and 42.2g of compound (D) to 300ml of dry dichloromethane under the protection of argon, stir, add 12.2g of triethylamine dropwise at 0°C, and then add 35.1g of compound (B) and dichloromethane dropwise 200ml of the prepared solution was stirred at 20°C for 1h, 300ml of saturated ammonium chloride solution was added to the reaction solution, the layers were separated, the aqueous phase was extracted with 200ml of dichloromethane × 2, the organic phases were combined, dried over anhydrous magnesium sulfate, and the dichloromethane was removed under reduced pressure. Chloromethane, column chromatography of the residue, eluent (n-hexane: ethyl acetate = 5: 1), to obtain 40.7 g of the target product (yield: 70.5%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com