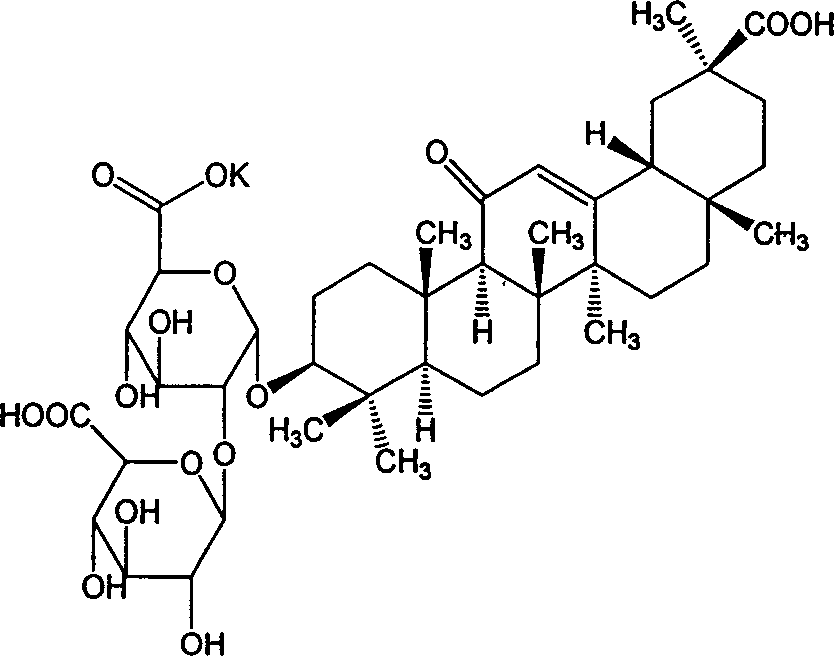
Film-coated tablet of glycyrrhizinic acid monopotassiium salt and method for preparing the same
A technology of glycyrrhizic acid monopotassium salt and film-coated tablets, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and pill delivery, etc. Uniformity, sugar-coated tablets take a long time to produce and other problems, to achieve the effect of improving bioavailability, improving appearance and stable properties of the main drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
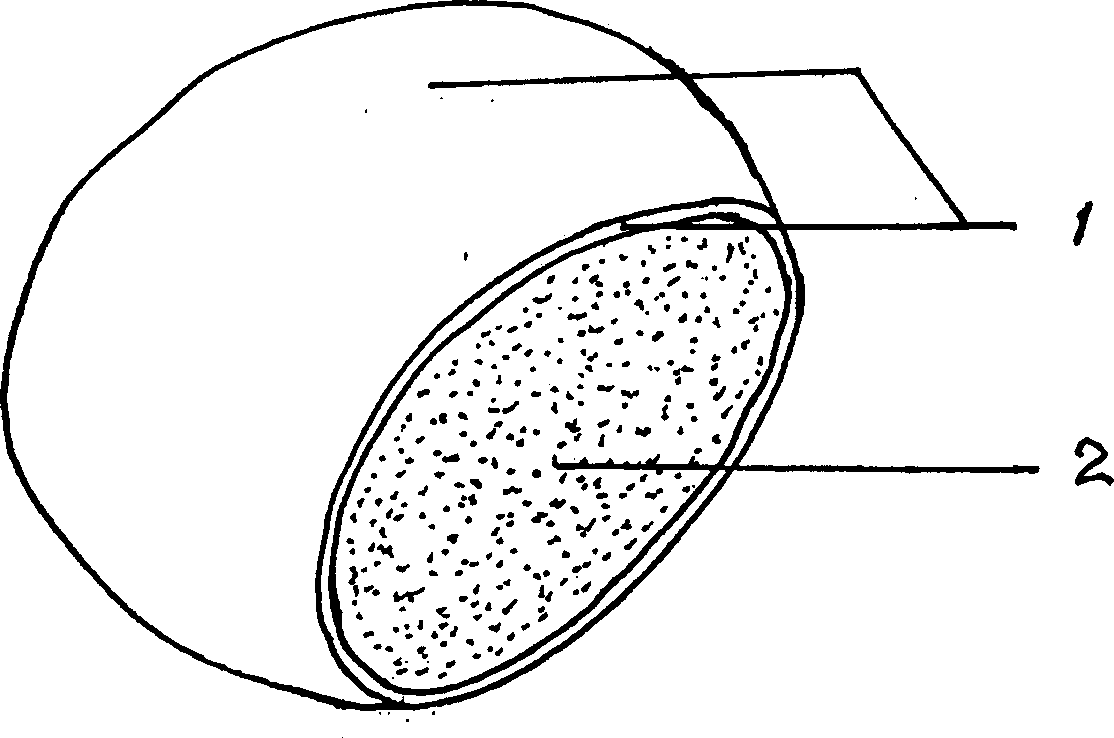
[0030] Glycyrrhizinic acid monopotassium salt film-coated tablet, with oblate glycyrrhizic acid monopotassium salt tablet with a diameter of 0.8-1.0 cm as the tablet base, the surface of the tablet is spray-coated with a layer of coating material film-coating layer with a thickness of 0.4-0.5 mm.
[0031] The components of the monopotassium glycyrrhizinate tablet are as follows: monopotassium glycyrrhizinate 5g, starch 50g, sucrose 40g, micronized silica gel 5g, magnesium stearate 120g, sodium carboxymethyl starch 130g, sodium lauryl sulfate 50g, 150g of talcum powder, 6g of alcohol 50% by volume. Coating material Opadry 100.0g was added with 900ml of ethanol with a volume percentage of 80% to make slurry.
[0032] The preparation method is as follows:
[0033] Preparation of oblate monopotassium glycyrrhizic acid tablets with a diameter of 0.8 to 1.0 cm: (1) crushing and sieving: the main drug monopotassium glycyrrhizic acid was passed through a 100 mesh sieve, magnesium ste...
Embodiment 2
[0036] Glycyrrhizic acid monopotassium salt film-coated tablet, with oblate glycyrrhizic acid monopotassium salt tablet with a diameter of 0.9-1.1 cm as the tablet base, the surface of the tablet is spray-coated with a layer of coating material film-coating layer with a thickness of 0.5-0.6 mm. (Such as figure 1 shown)
[0037] The components of the monopotassium glycyrrhizinate tablet are as follows: monopotassium glycyrrhizinate 8g, starch 80g, sucrose 70g, micropowder silica gel 8g, magnesium stearate 150g, sodium carboxymethyl starch 170g, sodium lauryl sulfate 80g, 180g of talcum powder, 8g of alcohol 50% by volume. 200.0 g of the coating material Opadry was mixed with 2000 ml of 80% ethanol by volume.
[0038] The preparation method is as follows:
[0039] Preparation of oblate monopotassium glycyrrhizic acid tablets with a diameter of 0.9 to 1.1 cm: (1) crushing and sieving: the main drug monopotassium glycyrrhizic acid was passed through a 100 mesh sieve, magnesium st...
Embodiment 3
[0042] Glycyrrhizic acid monopotassium salt film-coated tablet, with oblate glycyrrhizic acid monopotassium salt tablet with a diameter of 1.0-1.15 cm as the tablet base, the surface of the tablet is spray-coated with a layer of coating material film-coating layer with a thickness of 0.6-0.7 mm.
[0043] The components of the monopotassium glycyrrhizic acid tablet are as follows: monopotassium glycyrrhizinate 10g, starch 100g, sucrose 100g, micronized silica gel 10g, magnesium stearate 200g, sodium carboxymethyl starch 200g, sodium lauryl sulfate 100g, talcum powder 200g, 50% alcohol by volume percentage 10g. Coating material Opadry 300.0g, add 3600ml of ethanol with a volume percentage of 80% to prepare slurry.
[0044] The preparation method is as follows:
[0045] Preparation of oblate monopotassium glycyrrhizic acid tablets with a diameter of 1.0 to 1.15 cm: (1) crushing and sieving: the main drug monopotassium glycyrrhizic acid was passed through a 100 mesh, magnesium st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com