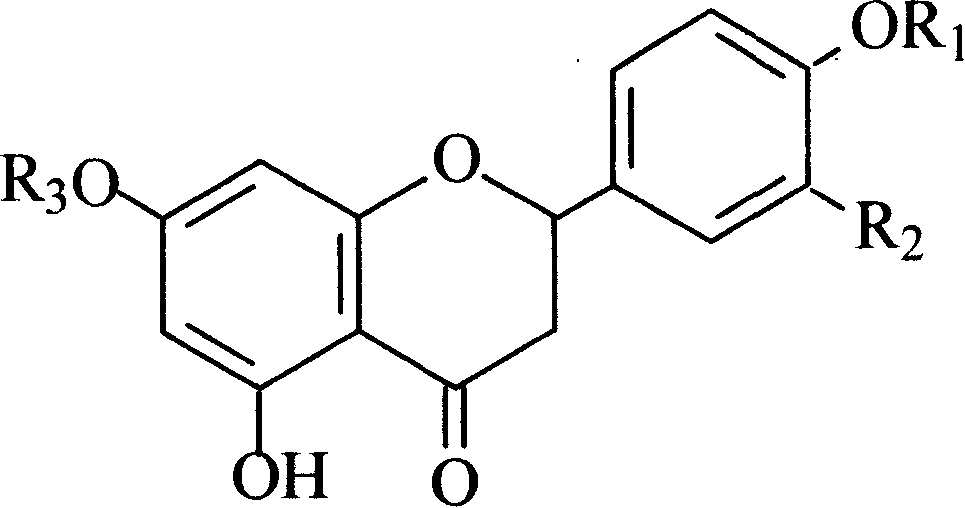
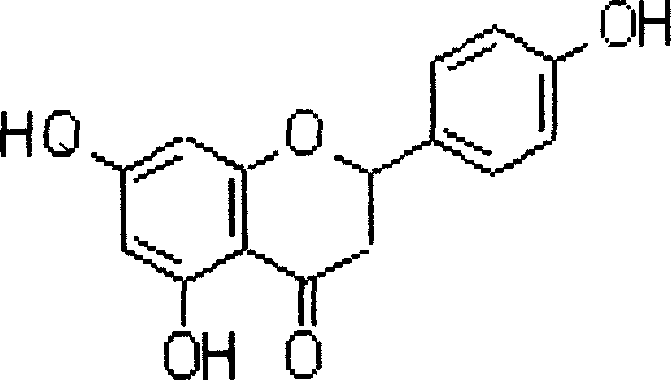
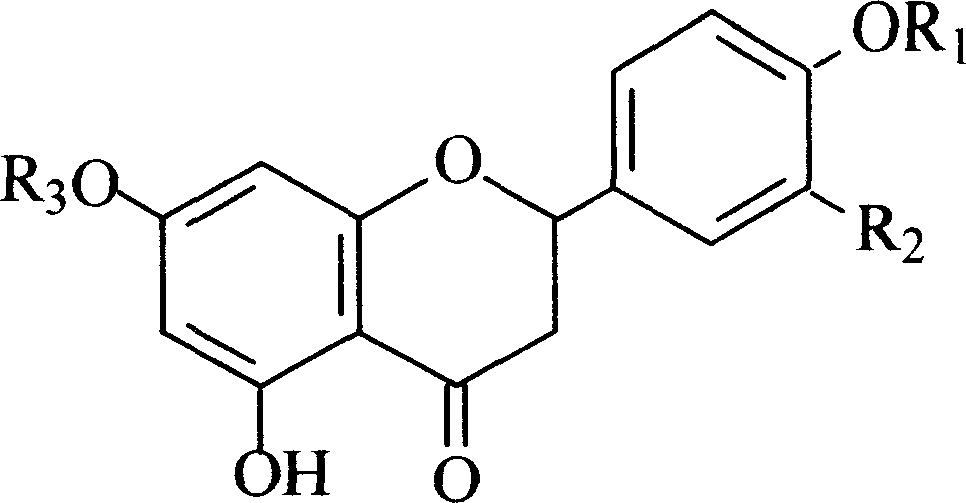
Use of naringenin and derivative in preparation of product for resisting cardiovascular and cerebrovascular disease
A technology for cardiovascular and cerebrovascular diseases and naringenin, which is applied in the fields of food and beverages and medicine, and can solve problems such as the influence of liver function and transaminase index, the growth of sales is not as good, and market development is restricted.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Embodiment 1, the impact of naringenin and its derivatives on acute cerebral ischemia in mice
[0189] grouping:
[0190] ①Blank control group: given the same volume of normal saline as the drug group;
[0191] ② Positive control drug group: Administer breviscapine tablets (Shanghai Leiyunshang Pharmaceutical Co., Ltd., product batch number 031111), with a dose of 200 mg / kg;
[0192] ③Test drug group: given the test drug, the dose is 200mg / kg;
[0193] method:
[0194] Each group was intragastrically administered once a day for 7 days. One hour after the last administration, the anesthetized mice were fixed in the supine position on the surgical closure, the skin of the neck was cut open, the common carotid arteries on both sides were separated, the left and right common carotid arteries with the vagus nerve were ligated with No. 4 surgical thread, and the death of the mice was recorded. time.
[0195] group
dosage
Average survival time of mice (mi...
Embodiment 2
[0198] Example 2. Protective effect of naringenin and its derivatives on focal cerebral ischemia-reperfusion injury in rats
[0199] Group:
[0200] ①Blank control group: given the same volume of normal saline as the drug group;
[0201] ② Positive control drug group: the drug breviscapine tablets (Shanghai Leiyunshang Pharmaceutical Co., Ltd., product batch number 031111) was administered at a dose of 50 mg / kg;
[0202] ③Test drug group: given the test drug, the dose is 50mg / kg;
[0203] method:
[0204] The SD rats, 280-300g, were anesthetized with 10% chloral hydrate 3ml / kg intraperitoneally, and the neck was cut in the middle, and the proximal end of the right common carotid artery, external carotid artery and its branch arteries were separated and ligated. The right internal carotid artery was separated, the pterygognathic artery was separated downward along the internal carotid artery, and the root of the branch was ligated. Prepare the thread at the proximal end of ...
Embodiment 3
[0212] Example 3, Effects of Naringenin and Its Derivatives on Learning and Memory in Vascular Dementia Rats
[0213] Group:
[0214] ①Blank control group: given the same volume of normal saline as the drug group;
[0215] ② Positive control drug group: the drug breviscapine tablets (Shanghai Leiyunshang Pharmaceutical Co., Ltd., product batch number 031111) was administered at a dose of 100 mg / kg;
[0216] ③Test drug group: given the test drug, the dose is 100mg / kg;
[0217] method:
[0218] The SD rats, 280-300g, were anesthetized with 10% chloral hydrate 3ml / kg intraperitoneally, and the neck was cut in the middle, and the proximal end of the right common carotid artery, external carotid artery and its branch arteries were separated and ligated. The right internal carotid artery was separated, the pterygognathic artery was separated downward along the internal carotid artery, and the root of the branch was ligated. Prepare the thread at the proximal end of the external ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com