Synthesis process of 18F lebeled positive electron radioactive tracer with ionic liquid as phase transfer catalyst
A technology of phase transfer catalyst and radioactive tracer, which is applied in radioactive carriers, organic chemical methods, chemical instruments and methods, etc. It can solve the problems of no water component and complicated labeling process, so as to improve work efficiency and shorten the synthesis process , the effect of improving the synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] 18 Synthesis steps of F-FDG:
[0079] 1. Will be transmitted from the accelerator 18 F ions and water pass to the receiving bottle (1-4Ci);
[0080] 2. will 18 F ions and water pass to the anion exchange column QMA;
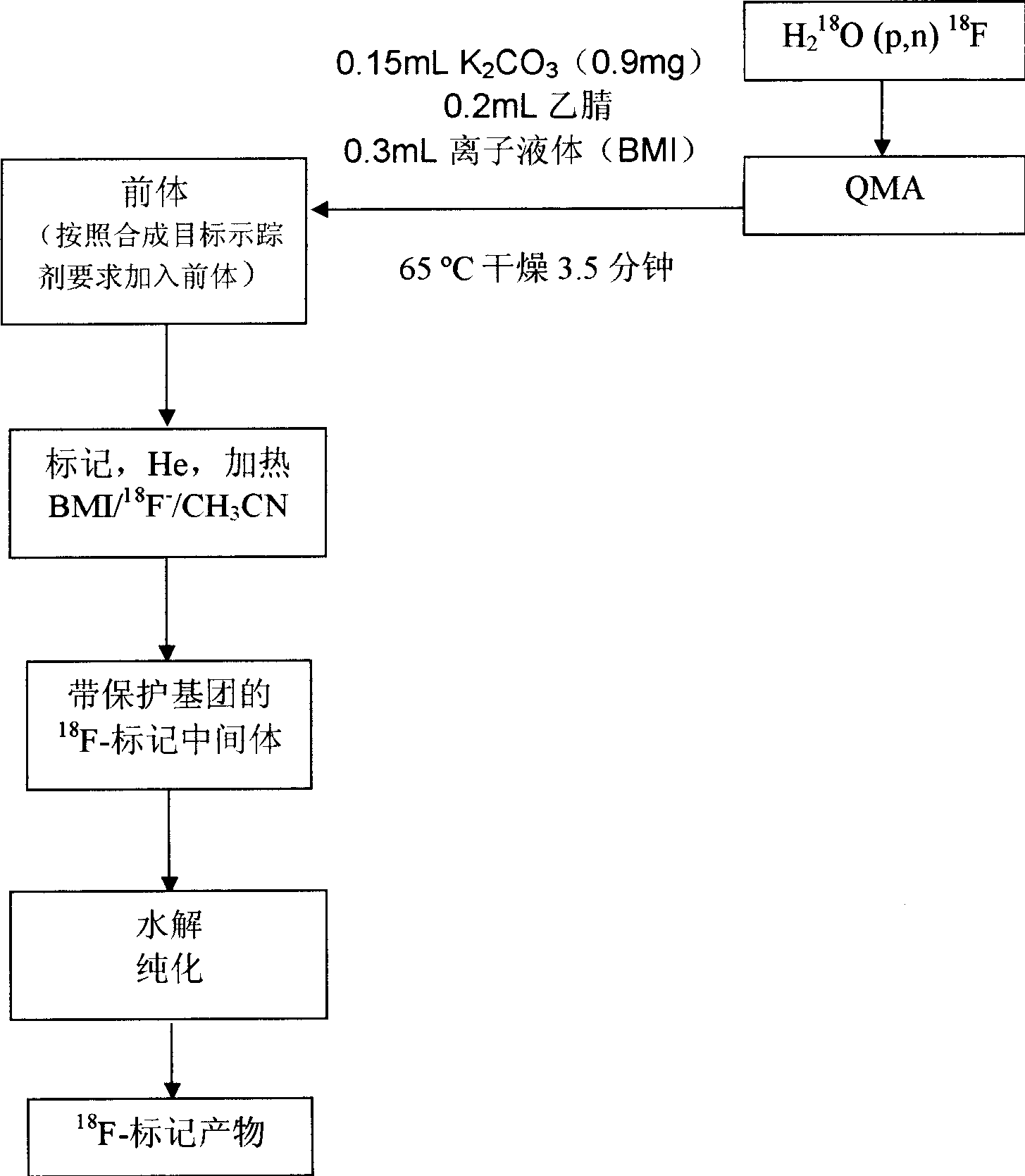
[0081] 3. Use 0.15mL K 2 CO 3 (0.9mg)+0.2mL acetonitrile+0.3mL ionic liquid (BMI) was eluted from the QMA column 18 F ions are sent to the reaction bottle; heated at 65 degrees for 3.5 minutes under the protection of nitrogen or helium;
[0082] 4. Dissolve the precursor (1,3,4,6-tetra-O-acetyl-2-O-trifluromethanesulfonyl-D-mannopyanose (TATM), 25mg) in 0.7mL acetonitrile, mix well and then add to the reaction bottle ;Heating at 100°C for 5 minutes under the protection of nitrogen or helium;
[0083] 5. Lower the temperature to 90°C, add 2ml of water to the reaction flask, cool to 33°C, then add 1M 1mL NaOH for 1.5 minutes to hydrolyze to remove the acetyl protecting group (AcO - );
[0084] 6. Pass the solution through a special FDG alkali hydro...
Embodiment 2
[0089] 18 Synthetic steps of F-FLT:
[0090] 1. Will be transmitted from the accelerator 18 F ions and water pass to the receiving bottle (1-4Ci);
[0091] 2. will 18 F ions and water pass to the anion exchange column QMA;
[0092] 3. Use 0.15mL K 2 CO 3 (0.9mg)+0.2mL acetonitrile+0.3mL ionic liquid (BMI) was eluted from the QMA column 18 F ions are sent to the reaction bottle; heated at 65 degrees for 3.5 minutes under the protection of nitrogen or helium;
[0093] 4. The precursor (3-N-tert-Butoxycarbonyl-(5'-O-(4,4'-dimethoxytripphenylmethyl)-2'-deoxy-3'-o-(4-nitrobenzenesulfonyl)-β-D-threopentofuranosyl )thymine, 30mg) was dissolved in 0.7mL acetonitrile, mixed well and then added to the reaction bottle; heated at 130°C for 15 minutes under the protection of nitrogen or helium;
[0094] 5. Lower the temperature to 100°C, add 1.2mL of 1N HCI to the reaction flask to react for 2 minutes, cool to 33°C, then add 1M 1mL NaOH;
[0095] 6. Pass the solution through an HP...
Embodiment 3
[0100] 18 The synthetic steps of F-ethylcholine:
[0101] 1. Will be transmitted from the accelerator 18 F ions and water pass to the receiving bottle (1-4Ci);
[0102] 2. will 18 F ions and water pass to the anion exchange column QMA;
[0103] 3. Use 0.15mL K 2 CO 3 (0.9mg)+0.2mL acetonitrile+0.3mL ionic liquid (BMI) was eluted from the QMA column 18 F ions are sent to the reaction bottle; heated at 65 degrees for 3.5 minutes under the protection of nitrogen or helium;
[0104] 4. Dissolve the precursor (1,2-bis(tosyloxy)ethane, 20mg) into 0.7mL acetonitrile, mix well and then add to the reaction bottle; heat at 100°C for 15 minutes under the protection of nitrogen or helium;
[0105] 5. When the temperature drops to 33°C, add 2mL eluent into the reaction bottle;
[0106] 6. The solution passes through two series-connected anion exchange columns (characteristics of the columns, hydroxide type) and finally enters the high pressure liquid chromatography (HPLC) column. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com