Metabolic protein for weed-control agent, its gene, and its use
A technology of protein and herbicide, applied in the field of protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0919] Example 1 Metabolism of Compound (II) by Microorganisms
[0920] (1) Metabolism of compound (II)
[0921] In ISP2 agar medium (1.0% (w / v) wort, 0.4% (w / v) yeast extract, 0.4% (w / v) glucose, 2.0% (w / v) agar, pH 7.3) Various microorganisms shown in Tables 1 and 2 were cultured. A loop amount of each microorganism was added to TGY medium (0.5% (w / v) tryptone, 0.5% (w / v) yeast extract, 0.1% (w / v) glucose, 0.01% (w / v) KH 2 PO 4 , pH 7.0) and incubated at 30°C with shaking for 2-4 days. One tenth milliliter (0.1 ml) of the obtained culture was added to 3 ml of sporulation broth (0.1% (w / v) meat extract, 0.2% (w / v) trypsin) containing 100 ppm of compound (II). % glucose, pH 7.1) for 7-8 days at 30°C with shaking. Fifty microliters (50 μl) of 2N HCl was added to the resulting culture and it was extracted with 3 ml of ethyl acetate. The ethyl acetate layer obtained was analyzed on HPLC. Decreasing the concentration of compound (II) (column retention time of 23.9 minutes)...
Embodiment 2
[0933] Example 2 Preparation of the protein (A1) of the present invention
[0934] (1) Preparation of crude cell extracts
[0935] A frozen stock of Streptomyces chromogenes IFO 12898 was added to 100 ml A medium (0.1% (w / v) glucose, 0.5% (w / v) tryptone, 0.5% (w / v) yeast) in a 500 ml Erlenmeyer flask Extract, 0.1% (w / v) dipotassium hydrogen phosphate, pH 7.0) and incubated at 30°C for 1 day with rotary shaking to obtain a pre-culture. Eight milliliters (8 ml) of the pre-culture was added to 200 ml A medium and incubated at 30°C for 2 days with rotary shaking in a 500 ml baffled shake flask. The cell pellet was recovered by centrifugation of the resulting culture (3,000 g, 5 min). The cell pellets were suspended in 100 ml B medium (1% (w / v) glucose, 0.1% beef extract, 0.2% (w / v) trypsin) containing 100 ppm compound (II) and incubated at 30°C. Incubate for 16 hours with reciprocating shaking in a 500 ml Sakaguchi flask. The cell pellet was recovered by centrifugation (3,000 ...
Embodiment 3
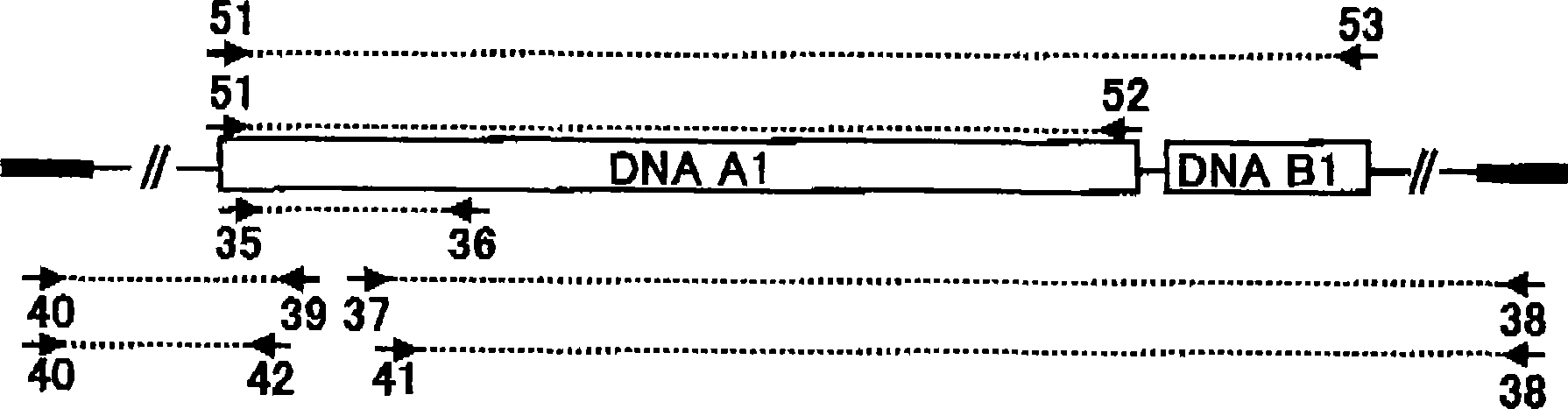
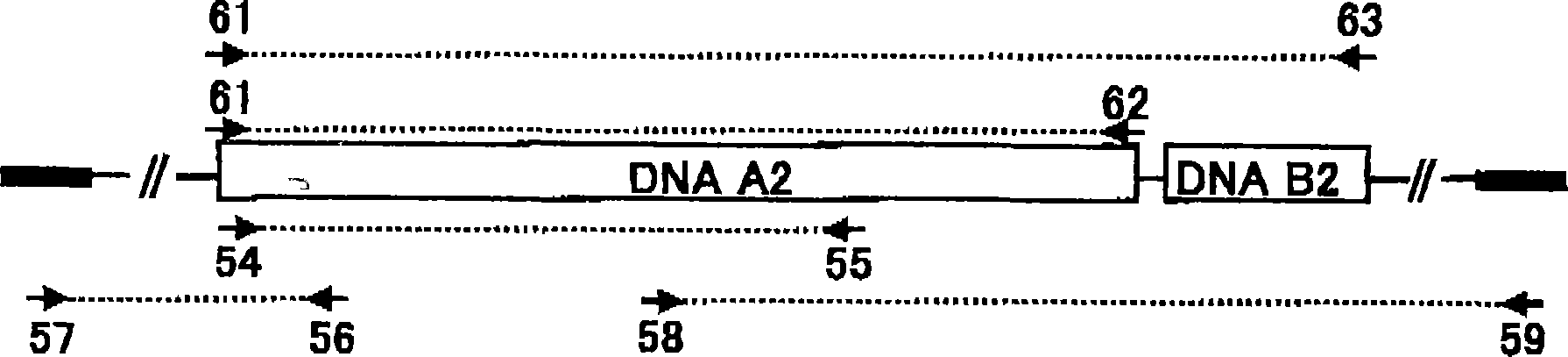
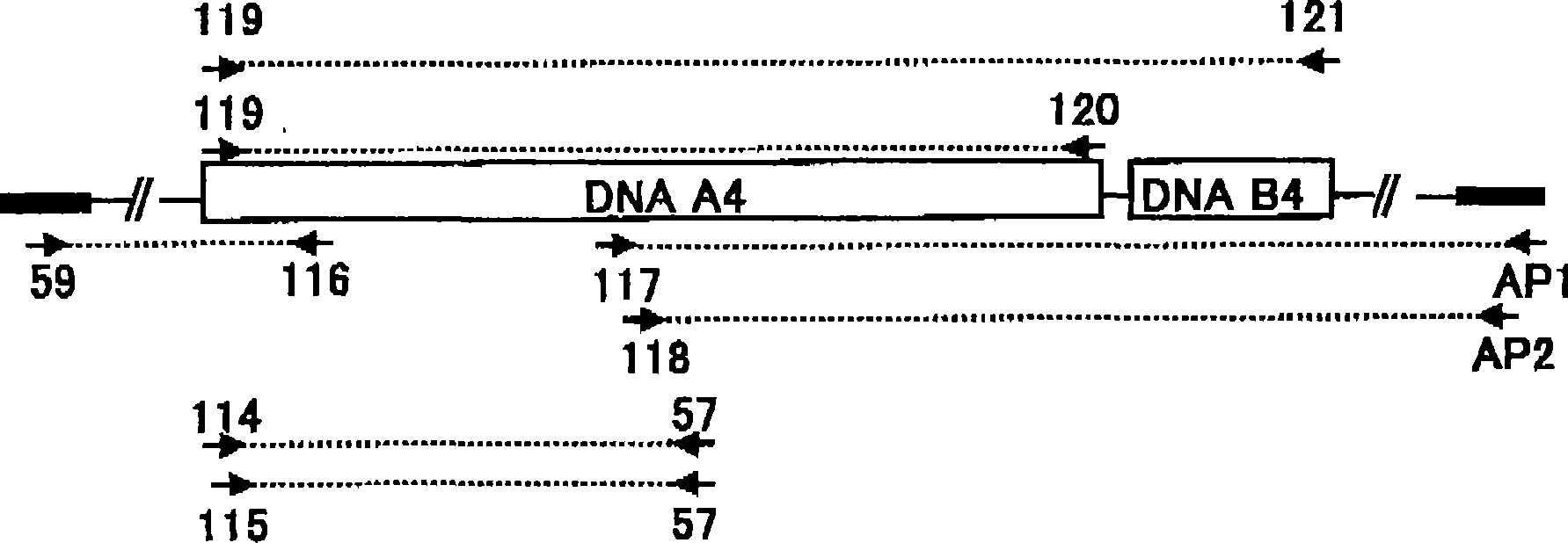
[0947] Example 3 Obtaining the DNA (A1) of the present invention
[0948] (1) Preparation of chromosomal DNA of Streptomyces dendrochromogenes IFO 12898
[0949] In 50 ml YEME medium (0.3% (w / v) yeast extract, 0.5% (w / v) peptone for bacteria, 0.3% (w / v) wort, 1.0% (w / v) glucose, 34% (w / v) w / v) sucrose and 0.2% (v / v) 2.5MgCl 2 ·6H 2 O) incubate Streptomyces chromogens IFO 12898 at 30°C for 1 to 3 days with shaking. Cells are recovered. The obtained cells were suspended in YEME medium containing 1.4% (w / v) glycine and 60 mM EDTA and further incubated with shaking for 1 day. Cells were recovered from the medium. After washing once with distilled water, it was resuspended in buffer (100 mM Tris-HCl (pH 8.0), 100 mM EDTA, 10 mM NaCl) at 1 ml per 200 mg of cells. Two hundred micrograms per milliliter (200 μg / ml) of egg white lysozyme was added. The cell suspension was incubated at 30°C for 1 hour with shaking. Additionally, 0.5% SDS and 1 mg / ml proteinase K were added. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com