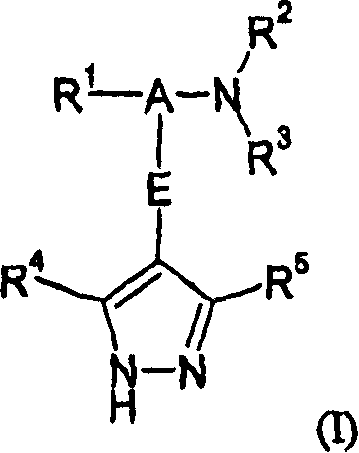
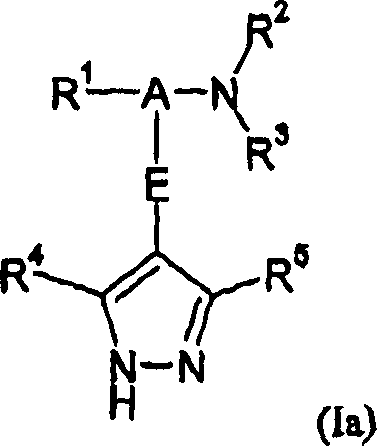
Pyrazole derivatives as protein kinase modulators
A technology of solvates and compounds, applied in the field of pyrazole derivatives as protein kinase regulators, can solve the problems of undisclosed inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0828] 2-Phenyl-2-[4-(1H-pyrazol-4-yl)-phenyl]-ethylamine
[0829]
[0830] To a suspension of 2-(4-chlorophenyl)-2-phenethylamine hydrochloride (134 mg, 0.5 mmol, 1.0 equiv) (Array PPA-Q02-1) in toluene (0.8 mL) was added bis( Tri-tert-butylphosphine)palladium(0) (3 mg, 1 mol%) (Strem), and the mixture was purged with nitrogen. Add 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (107 mg, 0.55 mmol, 1.1 equiv ) (Aldrich 52,505-7) in ethanol (0.8 mL), followed by the addition of potassium carbonate (415 mg, 3.0 mmol, 6 eq) in water (2.5 mL). The mixture was purged with nitrogen and sealed. Using 50 watts of power, the reaction mixture was placed in the CEM Explorer TM Heat to 135°C in microwave for 15 minutes. The solvent was removed and the residue was partitioned between ethyl acetate and 2N NaOH. The aqueous layer was extracted with ethyl acetate, and the combined organic layers were washed with brine, dried (MgSO 4 ), concentrated under reduced pressure...
Embodiment 2
[0832] 3-Phenyl-2-[3-(1H-pyrazol-4-yl)-phenyl]-propionitrile
[0833] 2A. 2-(3-Bromo-phenyl)-3-phenyl-propionitrile
[0834]
[0835] A solution of 40% KOH (2.83 g in 5.0 mL of water) in ethanol (13 mL) was added to benzaldehyde (2.85 mL, 28.05 mmol) and 3-bromophenylacetonitrile (5 g, 25.50 mmol) in ethanol ( 9 ml) solution. The reaction mixture was then stirred at room temperature for 2 hours and the precipitate was collected by suction filtration and washed with cold ethanol (6.68 g, 92%). The crude product (3.45 g, 12.14 mmol) was then dissolved in ethanol (35 mL) and heated to 65°C. Sodium borohydride (459 mg, 12.14 mmol) was added in several portions and the reaction mixture was maintained at this temperature for a further 2 hours. When cooled, water (10 mL) was added and the solvent was removed under reduced pressure. The residue was partitioned between water (100 mL) and ethyl acetate (100 mL). The organic layer was separated and dried (MgSO 4 ), filtered and ...
Embodiment 3
[0840] 2-[4-(3,5-Dimethyl-1H-pyrazol-4-yl)-phenyl]-2-phenyl-ethylamine
[0841]
[0842] Following the procedure of Example 1, but using 3,5-dimethyl-4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolane-2- Base)-1H-pyrazole (boron molecule D03-BM152) instead of 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)- 1H-Pyrazole to give the title compound. (LC / MS: (PS-A)R t 1.79[M+H] + 292.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com