Preparation of catalyst for synthesizing hydrotalcite thin film by alcohol oxidation reaction
A hydrotalcite, alcohol oxidation technology, applied in the preparation of organic compounds, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., can solve difficult reactants and reaction products, separation, subsequent activation Easy to fall off and other problems, to achieve uniform dispersion of wafers, increase contact area, and solve the effect of mass diffusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
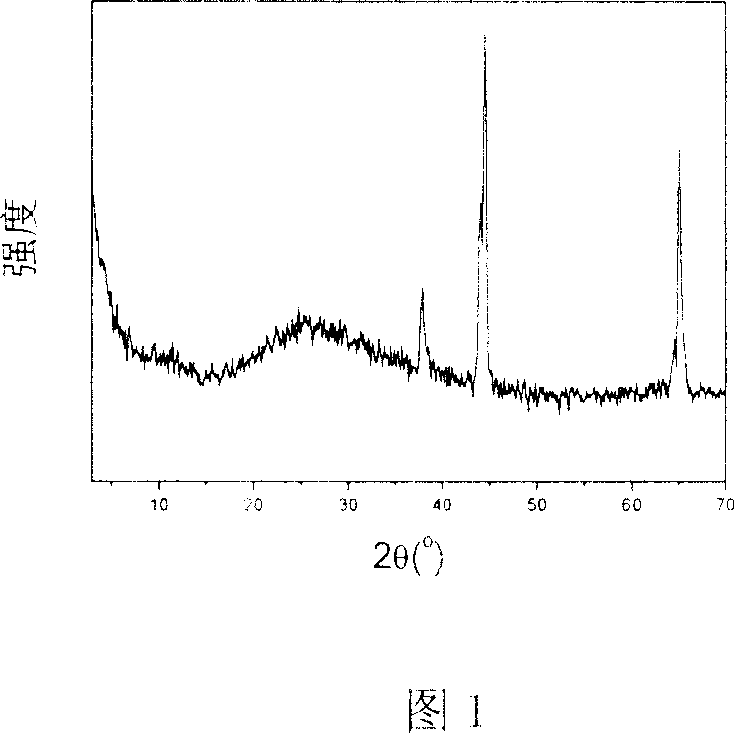
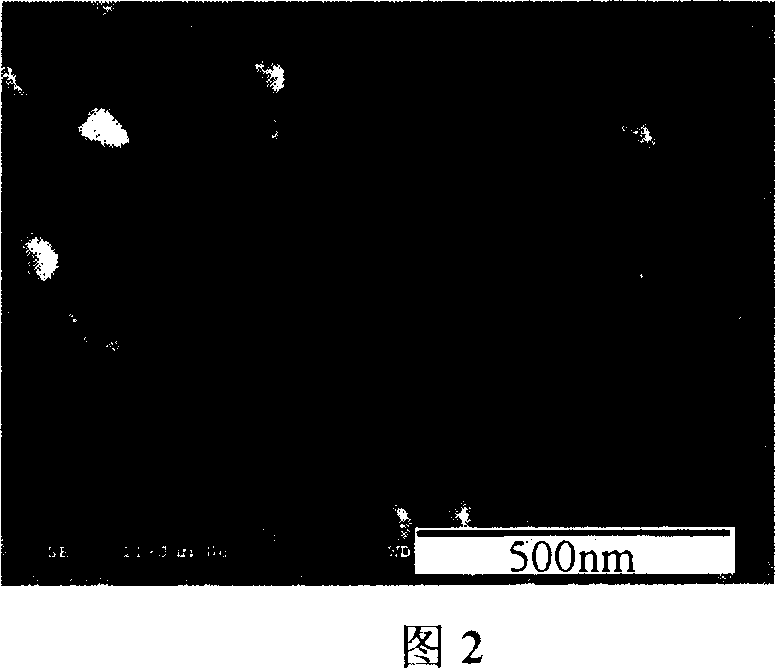
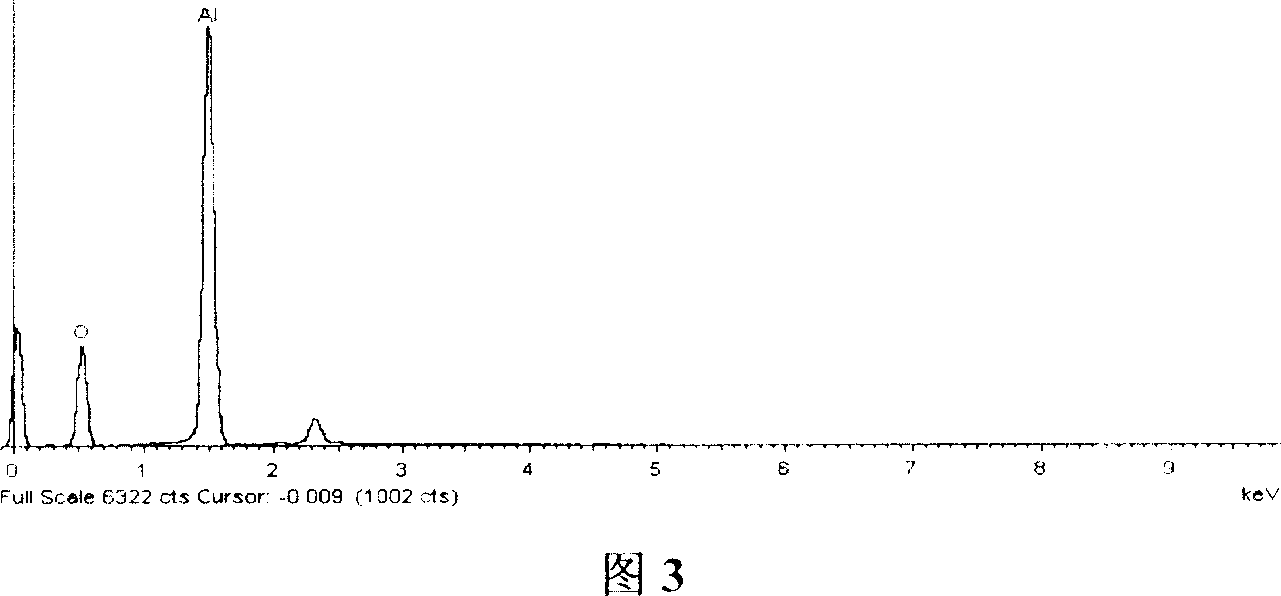
[0041] A 9.0cm×7.0cm aluminum sheet (purity 99.5%) was ultrasonically cleaned with absolute ethanol and deionized water for 3 minutes, and then the surface oxide layer was removed with 0.1mol / L sodium hydroxide solution, and then cleaned with deionized water Put it into an electrolytic cell as an anode, use a lead plate as a cathode, and use 1.0ml / L sulfuric acid solution as an electrolyte. The working voltage is 16V, and the oxidation current is 1.4A. Rinse with deionized water for later use. By X-ray powder diffractometer (XRD) (see Fig. 1), scanning electron microscope (SEM) (see Fig. 2), the characterization result of X-ray energy spectrometer (EDS) (see Fig. 3), show through anodic oxidation in aluminum A porous aluminum oxide film was obtained on the sheet surface.
[0042] 0.4365gNi(NO 3 ) 2 ·6H 2 O and 6.0030g NH 4 NO 3 Dissolve in 150ml of deionized water, pour into a 250ml three-neck flask, and prepare the Ni ion concentration as 0.01mol / L, NH 4 NO 3 A soluti...
Embodiment 2
[0045] The preparation method of aluminum base anodized aluminum is the same as embodiment 1
[0046] 0.4372gNi(NO 3 ) 2 ·6H 2 O and 6.0012g NH 4 NO 3 Dissolve in 150ml of deionized water, pour into a 250ml three-neck flask, and prepare the Ni ion concentration as 0.01mol / L, NH 4 NO 3 A solution with a concentration of 0.5mol / L, cut the aluminum sheet (6.5cm×4.5cm) with anodized surface into small pieces and put them into the solution, stir and impregnate at 50°C for 5h, then add ammonia water dropwise to adjust the pH value to 6.61,80 The reaction was stirred and reacted at ℃ for 24 hours. After the reaction solution was cooled, the film-grown substrate was taken out, washed 5 times with deionized water, and dried at 70 ℃ to obtain NiAlNO 3 - LDHs thin film catalyst.
[0047] The XRD spectrogram of the obtained LDHs thin film catalyst is shown in Figure 10, and the SEM and EDS spectrograms are shown in Figure 11 and Figure 12. The Ni / Al molar ratio in the LDHs film lay...
Embodiment 3
[0049] The preparation method of aluminum-based anodized aluminum is the same as that in Example 1.
[0050] 0.4360gCo(NO 3 ) 2 ·6H 2 O and 5.9999g NH 4 NO 3 Dissolved in 150ml deionized water, poured into a 250ml three-neck flask, and prepared to have a Co ion concentration of 0.01mol / L, NH 4 NO 3 A solution with a concentration of 0.5mol / L, cut the aluminum sheet (6.5cm×4.5cm) with anodized surface into small pieces and put them in the solution, stir and impregnate at 50°C for 5h, then add ammonia water dropwise to adjust the pH value to 7.05, 70 Stir the reaction at ℃ for 12h. After the reaction solution is cooled, take out the substrate with long film, wash it with deionized water for 5 times, and dry it at 70℃ to obtain CoAlCO 3 - LDHs thin film catalyst.
[0051] The XRD spectrogram of the obtained LDHs thin film catalyst is shown in Figure 13, and the SEM and EDS spectrograms are shown in Figure 14 and Figure 15. The Co / Al molar ratio in the LDHs film layer is 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com