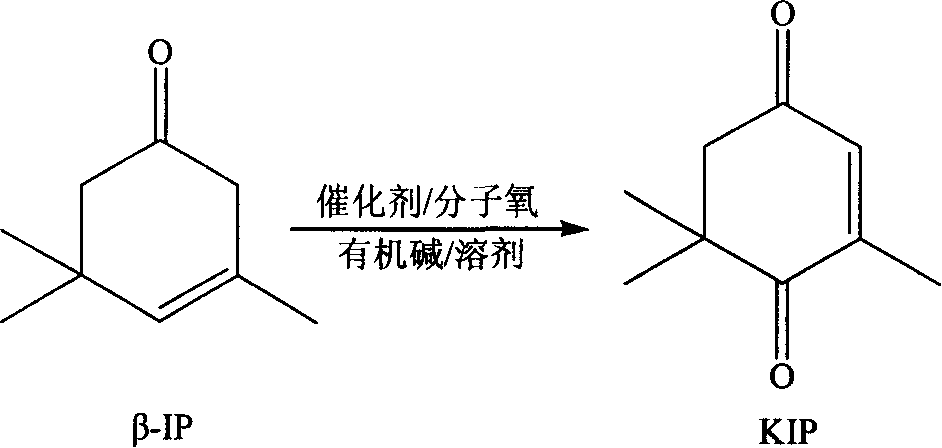
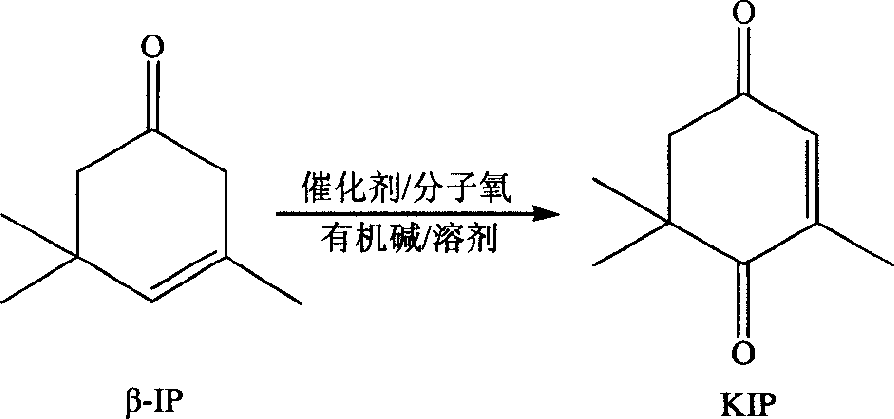
Method of preparing 3,5,5-trimethyl-cyclohex-2-en-1,4-dione by heterophase oxidation
A technology of trimethyl and cyclohexane, which is applied in the field of heterogeneous oxidation to prepare 3,5,5-trimethyl-cyclohex-2-ene-1,4-dione, which can solve the problem of reaction selectivity decline and separation Increased difficulty, high price, etc., to achieve the effects of improved reactivity and selectivity, easy separation and control, and easy reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 1g of catalyst (1#) and add it into a 250ml four-necked flask. Then add 50gβ-isophorone, 20ml pyridine and 30mlDMF successively, in a water bath at 80°C, feed air while vigorously stirring, the reaction is complete after 15 hours, the conversion rate is 98.2%, and the selectivity reaches 85.5%.
Embodiment 2
[0035] Weigh 2g of catalyst (2#) and add it into a 250ml four-necked bottle. Then add 50g beta-isophorone, 20ml pyridine and 30ml toluene successively, in water bath 60 ℃, pass into pure oxygen, stir vigorously simultaneously, after 18 hours, reaction completes, conversion rate is 98.7%, and selectivity reaches 88.5%.
Embodiment 3-10
[0037] implement
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com