Construction of HER2/neu mRNA in vitro transcription vector and use thereof
An in vitro transcription and carrier technology, applied in the fields of biology and medicine, can solve the problems of short half-life, obvious side effects, and inconvenient administration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 HER2 / neu 151-2220 Cloning of cDNA fragments
[0066] 1. Reverse transcription:
[0067] The HER2 / neu positive human breast cancer cell line SK-BR-3 (purchased from ATCC, No.: HTB-30) in good growth state was collected, and the total RNA of the cells was extracted with Trizol reagent (Invitrogen Company), and oligo-dT ( Under the guidance of Takara Company), AMV reverse transcriptase (Progema Company) was used to complete.
[0068] Add 1.2 μl of total RNA, 1.0 μl of oligo-dT and 7.8 μl of RNase-free water into a 200 μl RNase-free PCR reaction tube, mix well, centrifuge, place in a water bath at 65°C for 10 minutes, and quickly cool on ice for 5 minutes, then in the above reaction Add 4 μl of 5× buffer (AMV), 1 μl of 10 mM dNTP, 1 μl of AMV and 4 μl of RNase-free water to the system, mix well, incubate at 42°C for 1 hour, and then add 80 μl of water at 72°C for 10 minutes.
[0069] The reverse transcription product can be directly used as a template to amplif...
Embodiment 2
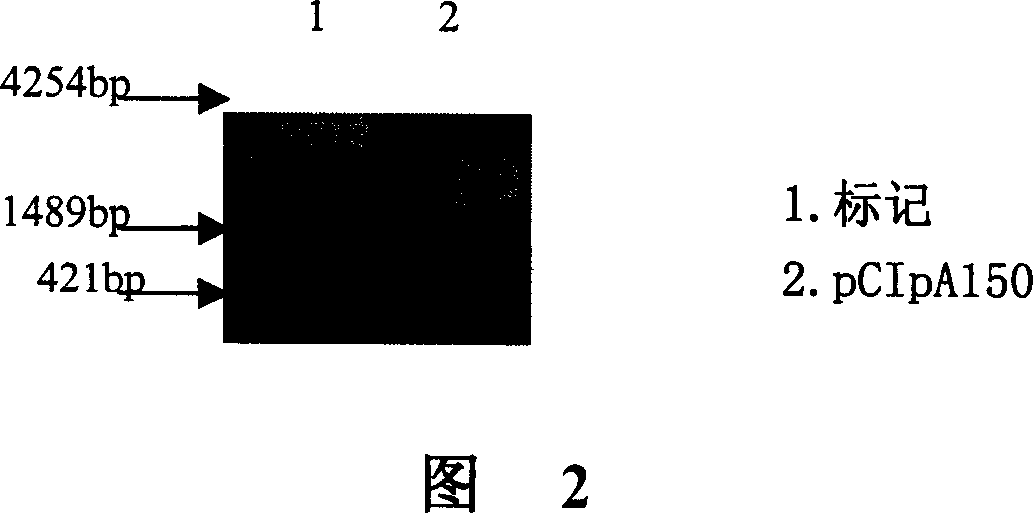
[0083] Example 2 Construction of in vitro transcription vector pCIpA150
[0084] 1. Amplification of polyadenylic acid (polyA)
[0085] A 150bp oligonucleotide polyA was amplified by PCR method.
[0086] Template: plasmid HNA6G1 (purchased from the Institute of Immunology, Second Military Medical University).
[0087] The sequence of the amplification primer (synthesized by Shanghai Sangon Biotechnology Co., Ltd.) is:
[0088] 5'-ACAGGCCTCCACTCCGTTTGCTG-3' (SEQ ID NO: 4) and
[0089] 5'-CCTCGAGGGATACTCTAGAGCG-3' (SEQ ID NO: 5).
[0090] In a 200 μl PCR reaction tube, add: 1 μl of plasmid (concentration: 100ng / μl), 4 μl of dNTP Mixture, 0.5 μl each of primers (concentration: 10 μM), 2.5 μl of 10×Pyrobest buffer, 0.125 μl of Pyrobest Enzyme and 16.375 μl of water. After mixing, complete the reaction on a PCR machine.
[0091] Reaction parameters: 95°C: 1 minute; 94°C: 30 seconds; 58°C: 30 seconds; 72°C: 30 seconds; 25 cycles; 72°C: 10 minutes.
[0092] The reaction product...
Embodiment 3
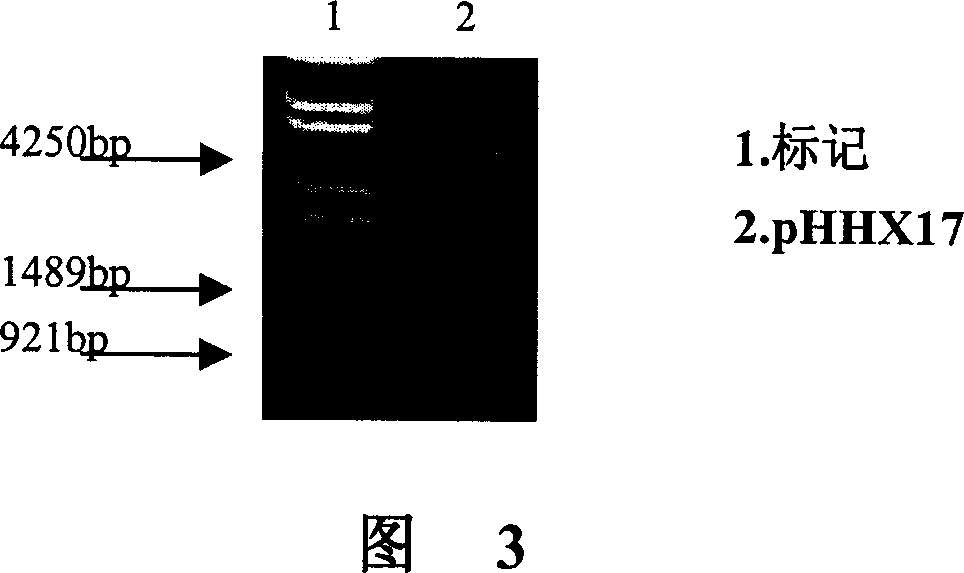
[0101] Example 3 Construction of HER2 / neu mRNA in vitro transcription vector pHHX17
[0102] 1. Enzyme digestion of vector pCIpA150
[0103] The vector pCIpA150 was double-digested with Xho I / Kpn I (Takara Company).
[0104]In a 1.5ml PCR reaction tube, add in sequence: 5 μl of small-scale prepared plasmid, 1 μl of 10× buffer M, 0.5 μl of Hpa I Enzyme, 0.5 μl of Kpn I Enzyme and 3 μl of water. Mix well, incubate at 37°C for 1.5 hours, perform 1% agarose gel electrophoresis, and recover the target fragment from the gel.
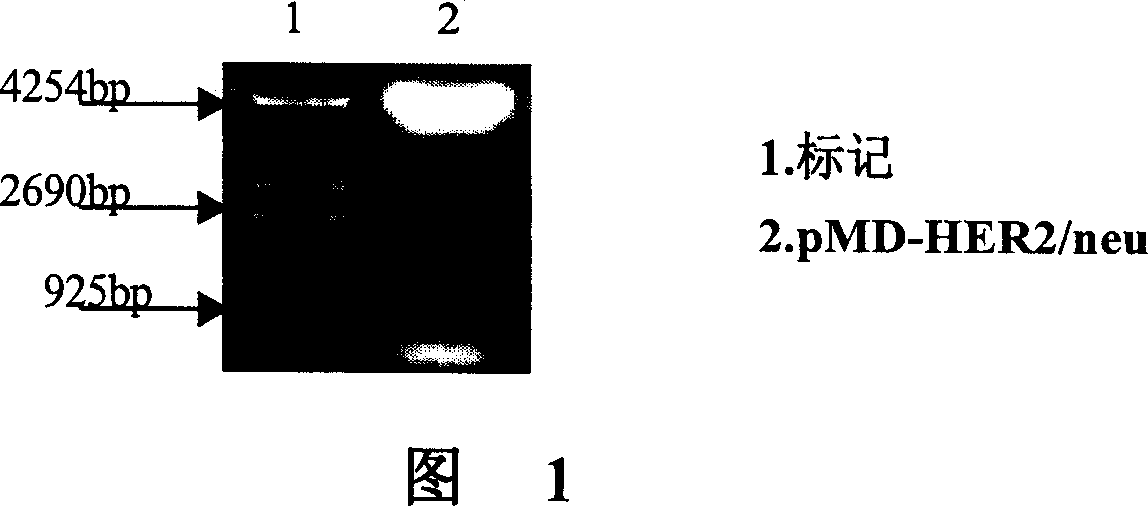
[0105] 2. Enzyme digestion of vector pMD-HER2 / neu
[0106] Double digestion with Kpn I and Sal I.
[0107] In a 1.5ml PCR reaction tube, add in sequence: 5 μl of miniprepared plasmid, 1.5 μl of 10× buffer T, 0.5 μl of Sal I Enzyme, 0.5 μl of Kpn I Enzyme, and 2.5 μl of water. Mix well, incubate at 37°C for 1.5 hours, perform 1% agarose gel electrophoresis, incubate at 37°C for 1.5 hours, and perform 1% agarose gel electrophoresis on the digested product. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com