Nasal administered preparation of melatonin
A technology for nasal administration preparations and melatonin, which is applied in the directions of antidote, drug combination, drug delivery, etc., can solve problems such as side reactions of nasal mucosa, and achieve the effect of no special odor.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 melatonin nasal spray (15mg melatonin / ml)
[0023] Melatonin 1.5g
[0024] PEG400 15ml
[0025] EDTA-2Na 0.01g
[0027] Ethyl p-hydroxybenzoate 0.1g
[0029] Add water for injection to 100ml
[0030] The clear yellow solution prepared according to the above prescription was divided into quantitative spray pumps to obtain nasal spray T1.
[0031] Also referring to PCT patent WO98 / 42333, the following prescription is designed:
[0032] Melatonin 1.5g
[0033] Hydroxypropyl Beta Cyclodextrin 13g
[0034] EDTA-2Na 0.01g
[0035] Vitamin C 0.2g
[0036] Benzalkonium Chloride 0.1g
[0038] Add water for injection to 100ml
[0039] The clear yellow solution prepared according to the above prescription was divided into quantitative spray pumps to obtain nasal spray T2.
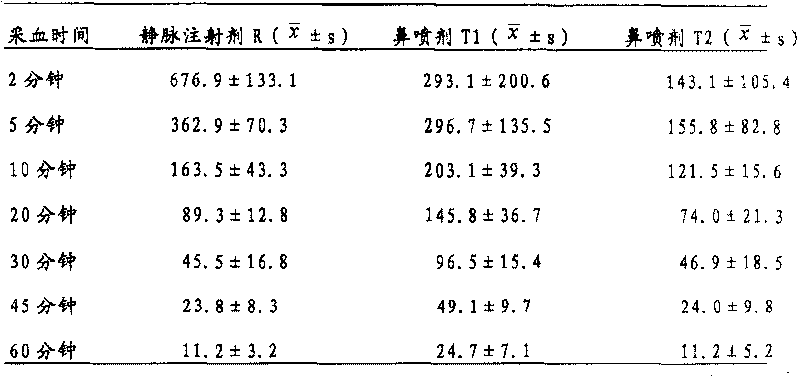
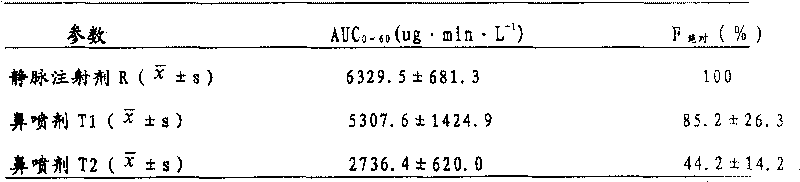
[0040] Another 3 mg / ml melatonin intravenous injection preparation R was prepared. Take 6 ...
Embodiment 2
[0048] Example 2 Melatonin nasal powder
[0049] Melatonin 1.5g
[0050] Lactose 30g
[0051] Sodium bisulfite 0.15g
[0052] Distilled water to 100ml
[0053] Add the above-mentioned melatonin and lactose into 100ml of distilled water, stir until completely dissolved, freeze-dry at -35°C overnight, then freeze-dry at -10°C and 0°C for 24 hours, pass through a 100-mesh sieve for nasal inhalation.
Embodiment 3
[0054] Embodiment 3 melatonin microsphere preparation
[0055] Melatonin 1.5g
[0056] Span 80 1.0g
[0057] Gelatin 6.0g
[0058] Medicinal castor oil 100ml
[0059] Toluene solution of saturated glutaraldehyde Appropriate amount
[0060] Take the above gelatin and dissolve it in 30ml of 55°C water, add melatonin to dissolve it. Separately take medicinal castor oil and emulsifier Span80 in a dry beaker, place the beaker in a water bath at 50°C, and slowly add it into the gelatin solution containing the drug under stirring at a stirring speed of 1000 rpm. After stirring for 10 min, a saturated toluene solution of glutaraldehyde was added, stirring was continued for 2 h, and the mixture was washed with n-hexane:isopropanol (1:1). The microspheres obtained by centrifugation were washed with ether, dried, sieved and packaged for use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com