Manganese catalyst of 8-hydroxy quinoline derivative and its uses in olefin epoxidation
A hydroxyquinoline, manganese catalyst technology, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, organic chemistry and other directions, can solve the problem of low practical value, difficult synthesis, difficult recovery, etc. problem, to achieve the effect of high utilization rate, high epoxidation efficiency and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
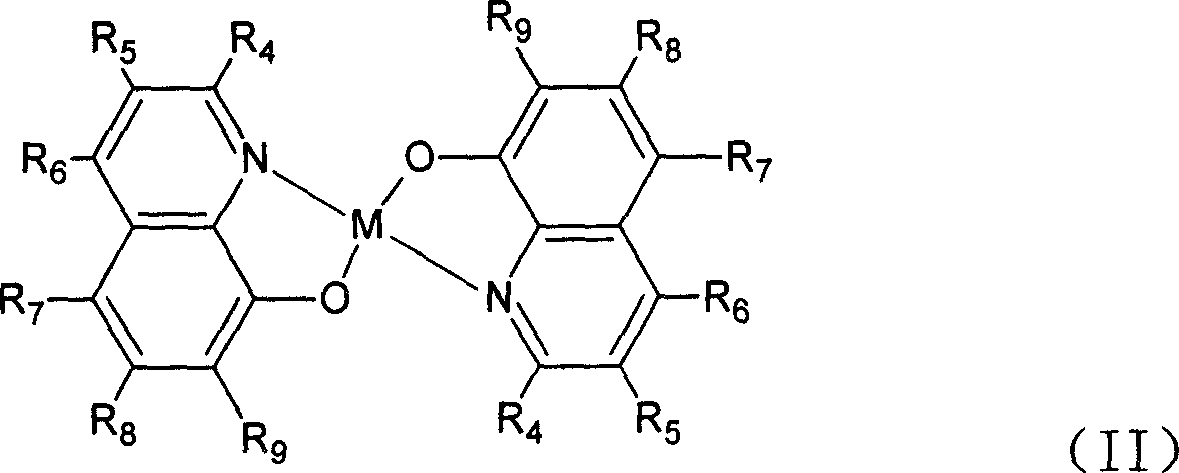
[0027] Embodiment 1: the synthesis of general formula (II) catalyst:
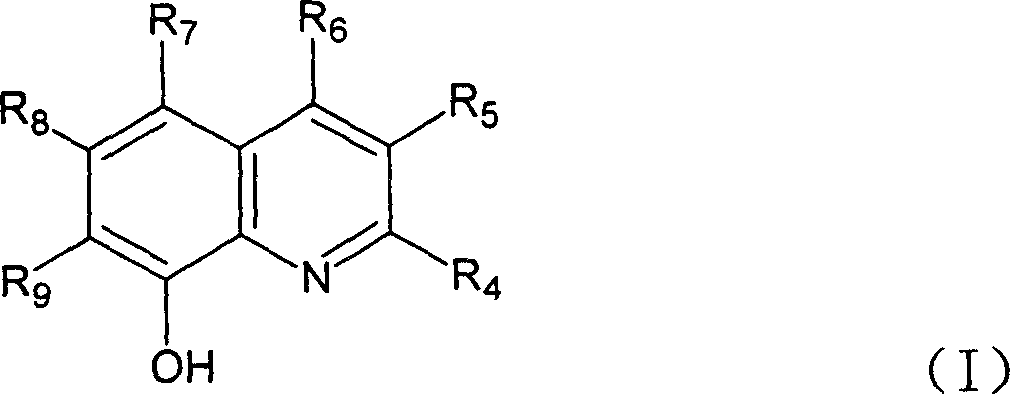
[0028] Dissolve 10mmol of the ligand of general formula (I) in 30-100ml of ethanol or methylene chloride, and add 20ml of Mn(OAc) containing 5mmol Mn(OAc) dropwise under stirring. 2 ethanol solution. Continue stirring at room temperature for 10 to 20 minutes. Filter with suction, wash with ethanol, and dry to obtain a solid that is the catalyst of general formula (II). The product yield is about 90%.
Embodiment 2
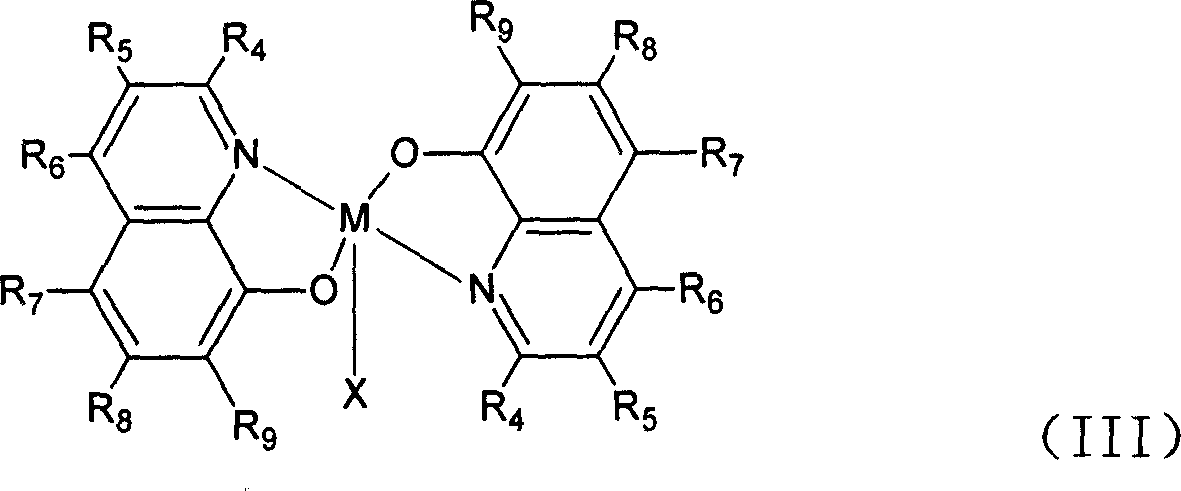
[0029] Embodiment 2: the synthesis of general formula (III) catalyst:
[0030] Dissolve 10mmol of the ligand of general formula (I) in 30-50ml
[0031] In tetrahydrofuran, add 20ml of 5mmol MnCl dropwise under stirring 2 ethanol solution. Then drop in 2-3mmol hydrogen peroxide, then drop in ammonia water to adjust the pH value of the solution to between 6-7 or drop in 4ml containing 10mmolNH 4 Aqueous solution of OAc. Continue to stir or reflux at room temperature for 1 to 2 hours. Filtrate with suction, wash with ethanol, and dry to obtain a catalyst (X=Cl) of general formula (III). The product yield is about 90%.
Embodiment 3
[0032] Embodiment 3: the synthesis of general formula (III) catalyst:
[0033] Dissolve 10mmol of a ligand of general formula (I) in 30-50ml of tetrahydrofuran, and add 20ml of Mn(OAc) containing 5mmol Mn(OAc) dropwise under stirring. 2 ethanol solution. Then add 2-3mmol hydrogen peroxide dropwise; continue to stir or reflux at room temperature for 1 to 2 hours; filter with suction, wash with ethanol, and dry to obtain a solid that is the desired catalyst (X=OAc); the product yield is about 90% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com