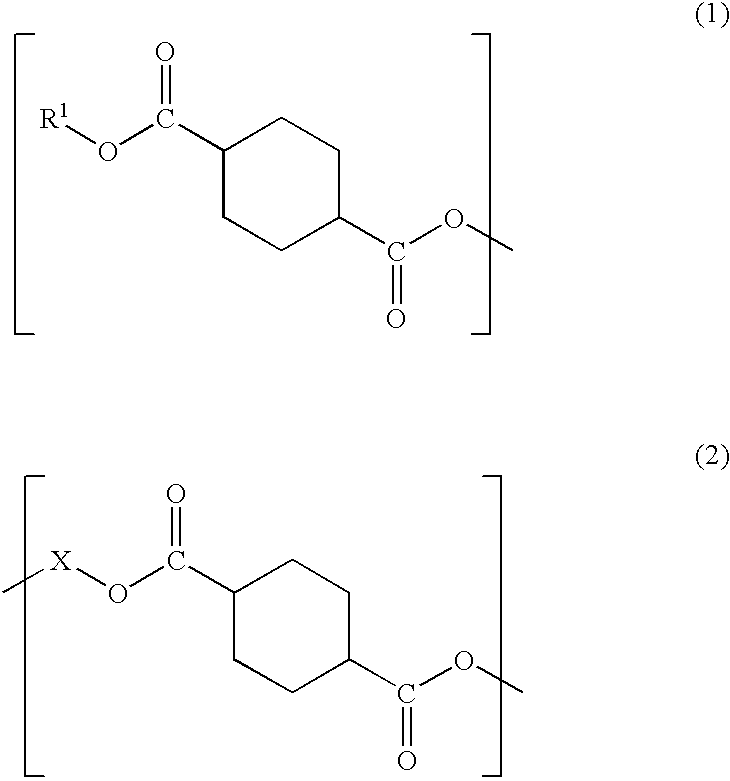
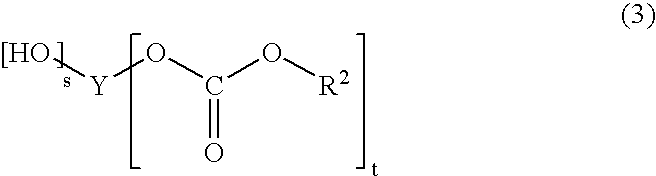
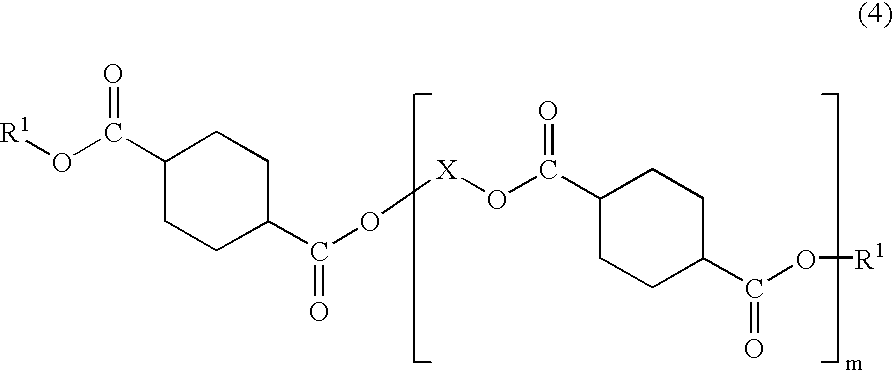
Plastic lens material, production process of the material, composition for plastic lens, plastic lens obtained by curing the composition, and production process of the plastic lens
a technology of plastic lens and composition, which is applied in the field of plastic lens material, can solve the problems of insufficient high value, low refractive index, and disadvantageous inferiority of plastic lens derived from polycarbonate resin in solvent resistance and scratch resistance, and achieve the effect of improving the crystallinity of diallyl ester oligomer and improving the releasability of plastic lens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0158] As shown in Table 3, 95.0 parts by mass of diethylene glycol bis(allyl carbonate) (CR-39, a trade name, produced by PPG), 5.0 parts by mass of Sample F and 3 parts by mass of diisopropylperoxy dicarbonate (IPP) were blended and mixed with stirring to form a completely homogeneous solution composition. The viscosity at this time was measured. Thereafter, the vessel containing this solution was placed in a desiccator capable of depressurization and the pressure was reduced by a vacuum pump for about 15 minutes to deaerate gases in the solution. The resulting solution composition was injected by a syringe into a mold fabricated from a glass-made mold for ophthalmic plastic lenses and a resin-made gasket, while taking care to prevent mixing of gas, and then cured in an oven according to a temperature rising program such that heating at 40.degree. C. for 7 hours, heating from 40.degree. C. to 60.degree. C. for 10 hours, heating from 60.degree. C. to 80.degree. C. for 3 hours, heat...
examples 2 to 5
[0160] EXAMPLES 2 TO 5 and Comparative Examples 1 and 2
[0161] Compositions were prepared according to the proportions blending shown in Table 2 and the viscosity was measured in the same manner as in Example 1. After the curing, the compositions were measured for the refractive index, Abbe number and Barcol hardness, and evaluated for the uneven dyeing. The results are shown in Table 2.
[0162] Change in Appearance (Storage Stability Test)
[0163] Samples F, G, H and I were stored at 25.degree. C. for 2 weeks and the appearance was visually compared between the sample just after the production and the sample after storage for two weeks. The results are shown in Table 3. Also, by blending Sample F, G, H or I just after the production or after the storage for two weeks, resin compositions for a plastic lens were prepared and the appearance thereof was compared in the same manner. The results are shown in Table 4.
4 TABLE 3 Sample F Sample G Sample H Sample I Appearance transparent transpar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass % | aaaaa | aaaaa |
| mass % | aaaaa | aaaaa |
| mass % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com