Low energy chlorate electrolytic cell and process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-4
Forming No Part of this Invention
[0027] In order to demonstrate the reduced power usage that can be expected where a precious metal oxide coated cathode is substituted for the titanium or mild steel cathodes presently utilized in commercial sodium chlorate electrolysis cells, a comparison was made of these cathodes as well as an oxygen reduction cathode by measuring the single electrode potential of these cathode materials against a saturated calomel reference electrode. The conditions for measuring the single electrode potentials of these cathode materials were as follows: Sodium hydroxide electrolyte—one molar, current density—one amp per square inch, temperature—30 to 40 degrees centigrade. Where an oxygen reduction cathode was used, pure oxygen was fed to the cathode. Cathodes of titanium and mild steel, represent commercial cathodes utilized for the production of sodium chlorate. The precious metal oxide coated cathode was a mixture of platinum and ruthenium oxides thermally d...
example 5
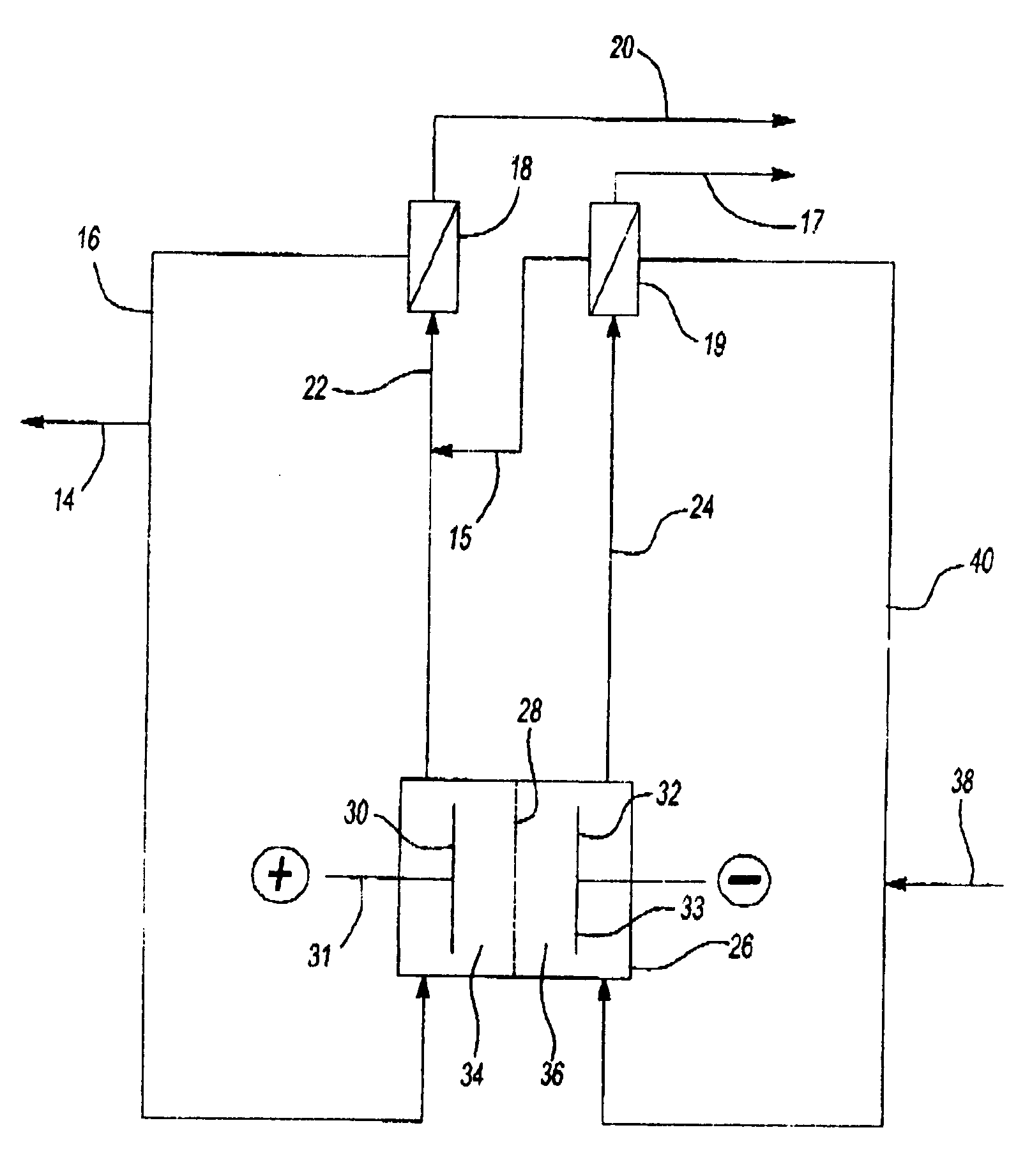
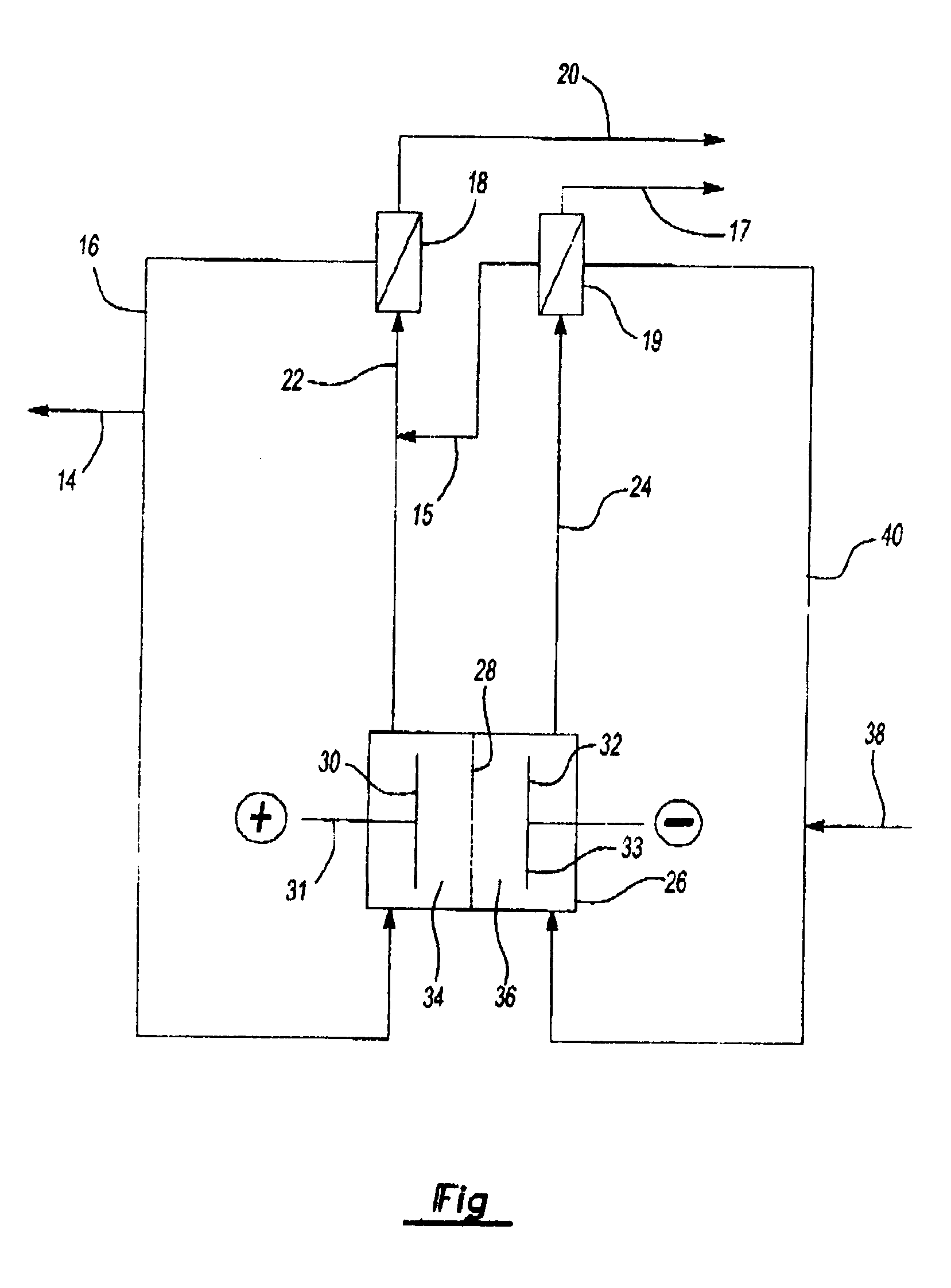
[0036] A pilot plant size plate and frame type electrolytic cell electrolysis process for the production of sodium chlorate is described in which the anode and cathode compartments are separated by a microporous diaphragm.
[0037] The anode used in the cell was a platinum-iridium oxide coated titanium expanded mesh substrate. The activated cathode was a platinum-ruthenium oxide coated nickel expanded mesh substrate. The electrode size was 3 inches by 10 inches providing a total of 30 square inches of active surface area. The microporous diaphragm utilized to separate the anolyte and catholyte compartments of the cell was a hydrophilic polyvinylidene fluoride sheet sold under the trademark Duropore® by the Millipore Corporation. This diaphragm had a pore diameter of about 0.1 micron, a thickness of 110 plus or minus 30 microns, and a porosity of 70%.
[0038] During the operation of the cell, the current input was measured at 35 amps or 1.17 amps per square inch current density. The tem...
example 6
[0041] Example 5 was repeated, except that in the process, using a plate and frame type electrolytic cell, an hydrophilic, polytetrafluoroethylene, microporous diaphragm sold under the trademark Advantec® by Advantec MSF, Inc. was used to separate the anode and cathode compartments of the cell.
[0042] The same cathode and anode as those described in Example 5 were used. The diaphragm pore diameter was 0.1 micron, the thickness was 25 plus or minus 1 microns, and the porosity was 71%. The current input was 35 amps or 1.17 amps per square inch current density. The temperature of both the anolyte and catholyte solutions was maintained at about 65 degrees centigrade by steam heat exchangers. The differential hydraulic pressure from the catholyte to the anolyte compartment was maintained at 4 inches of water.
[0043] In the process, purified brine was first acidified to a pH of about 3.5 to remove carbonate ions before being fed, at a rate of about 1.56 milliliters per minute, to the cath...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com