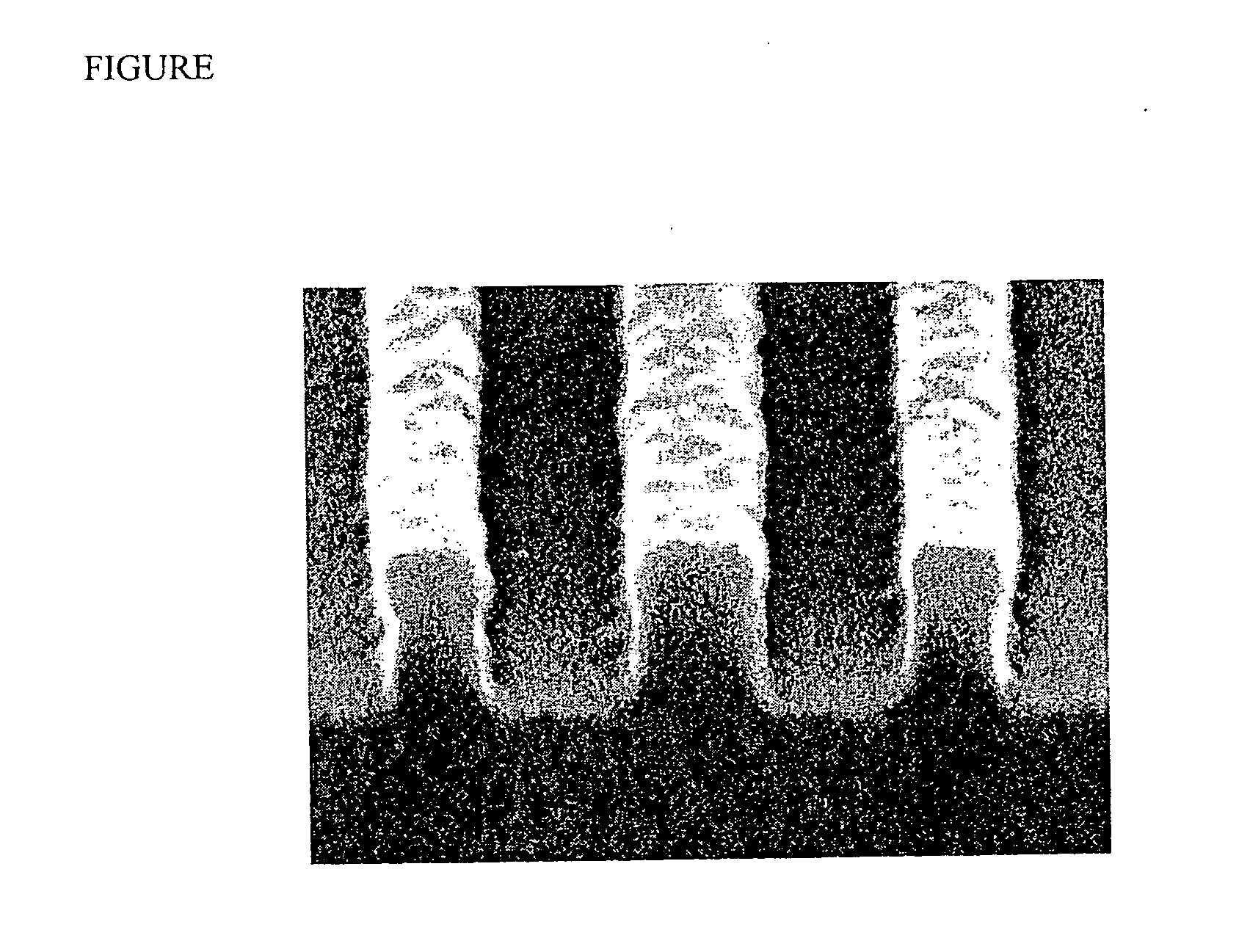
Photoimageable composition
a composition and composition technology, applied in the field of photoimageable compositions, can solve the problems of increasing resist thickness over diffusion steps on substrates, affecting the depth of focus of exposure tools, and affecting the accuracy of etching patterns, so as to achieve the effect of improving lithographic performance and lowering dissolution rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Poly(4-hydroxybenzyl silsesquioxane) (254.7 g) is dissolved in 1000 mL dry acetone under nitrogen atmosphere in a dried 3 L flask (reactor). Methanesulfonyl chloride (23.8 g) is added and the reactor is cooled to 15° C. A solution of distilled triethylamine (21.9 g) and acetone (22 g) is gradually added dropwise over 20-30 minutes, maintaining a reaction temperature of less than 30° C. Stirring is continued for 3 hours, at which time the solution is added dropwise over 2 h. to 32 L of water, precipitating the polymer. The polymer is then collected by suction filtration, and suspended in 8 L of water with stirring at room temperature for 18 h. The solid is then collected by suction filtration, is washed with water until the effluent is pH neutral, air-dried for 48 h., and is then dried in vacuo for 24 h. at 70° C. to yield an off-white polymer, having the formula 95 mol % hydroxybenzylsilsesquioxane / 5 mol % mesylatedbenzylsilsesquioxane. Yield: 246 g (85% of theory). GPC data (RI de...
example 2
A polymer with a higher mesylation level of 22% per molar equivalent of hydroxybenzyl group is prepared by the method of Example 1, except that the following reagent and solvent proportions are used: poly(4-hydroxybenzyl silsesquioxane) (63.7 g) in 250 mL dry acetone; methanesulfonyl chloride (13.8 g) in 9.3 g acetone; and triethylamine (12.6 g) in 17.4 g acetone. A 1 L reaction flask is used. Workup volumes are 8 L deionized water for precipitation, and 2 L deionized water for slurrying. The resulting polymer has the formula 78 mol % hydroxybenzylsilsesquioxane / 22 mol % mesylatedbenzylsilsesquioxane. Yield: 61 g (71% of theory); GPC (RI detection): Mw=5,571, Mn=3,456; molecular weight polydispersity=1.61. Tg=97° C. Dissolution Rate (0.26 N TMAH)=5,591 Å / sec. The isolated polymer is typically between 21-23% methanesulfonated, as determined by 1H NMR and as shown by the following general formula where x=0.79-0.77 and y=0.21-0.23.
example 3
5% Mesylated poly(4-hydroxybenzyl silsesquioxane) (163.1 g) from Example 1 is dissolved in 750 mL dry acetone under nitrogen atmosphere in a dried 2 L flask (reactor). Di-t-butyl dicarbonate (65.5 g) is dissolved in 300 mL acetone and added to the reactor, followed by N,N-dimethylaminopyridine (“DMAP”, 0.25 g) dissolved in 2 mL acetone, and the resulting pale orange solution is stirred 25° C. for 25 h. The acetone solution of polymer is added dropwise over 2 h. to 24 L of water, precipitating the polymer. The polymer is then collected by suction filtration, is washed with water, and dried in vacuo at 20° C. to constant weight (ca. 72 h.) to yield an off-white polymer, having the formula 65 mol % hydroxybenzylsilsesquioxane / 5 mol % mesylatedbenzylsilsesquioxane / 30 mol % tert-butoxycarbonato benzylsilsesquioxane as shown by the following general formula where x=0.65, y=0.05 and z=0.3. Yield: 174 g (90% of theory). GPC data (RI detection): Mw=6,216; Mn=3,636; molecular weight polydisp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelengths | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| optical density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com