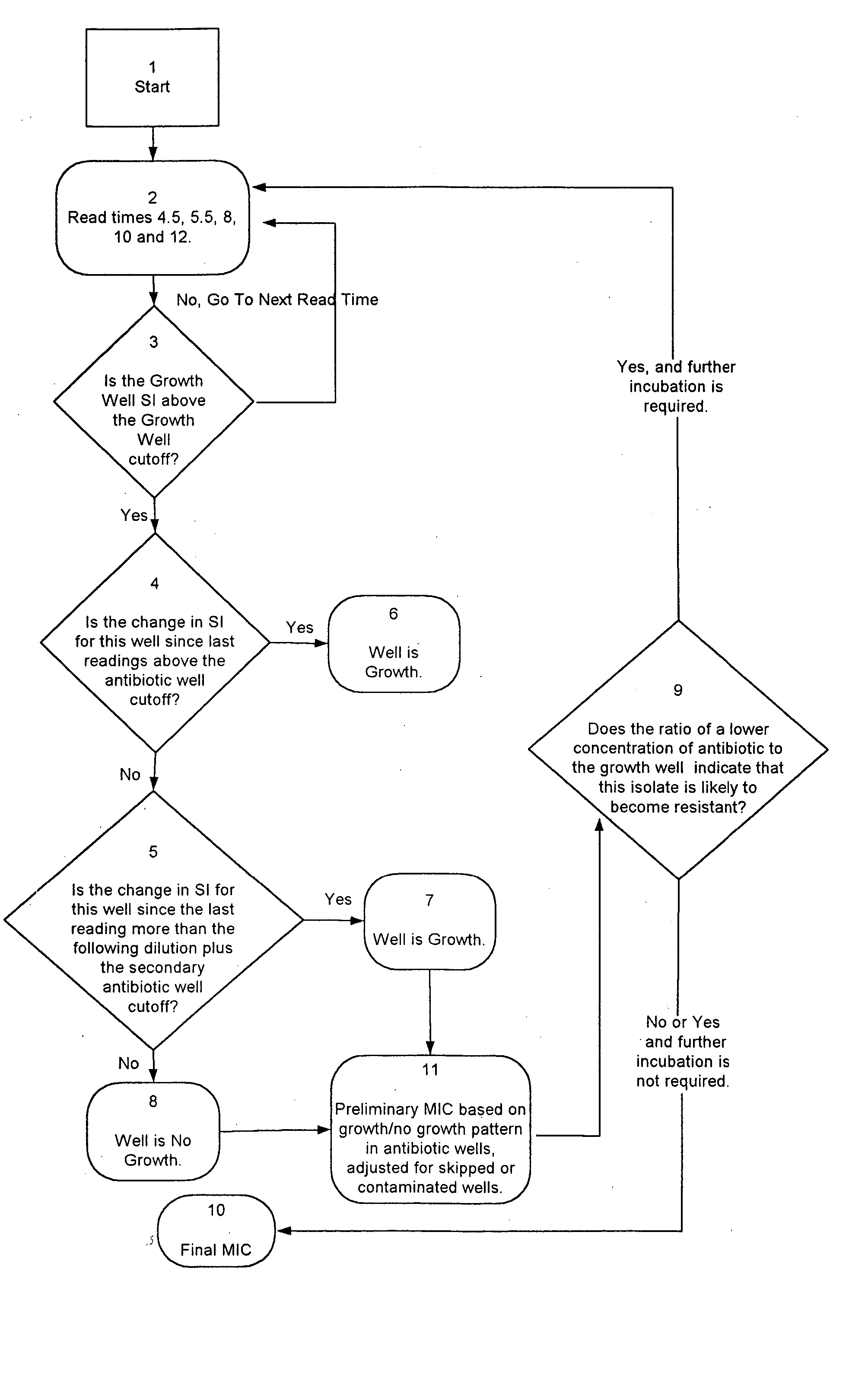
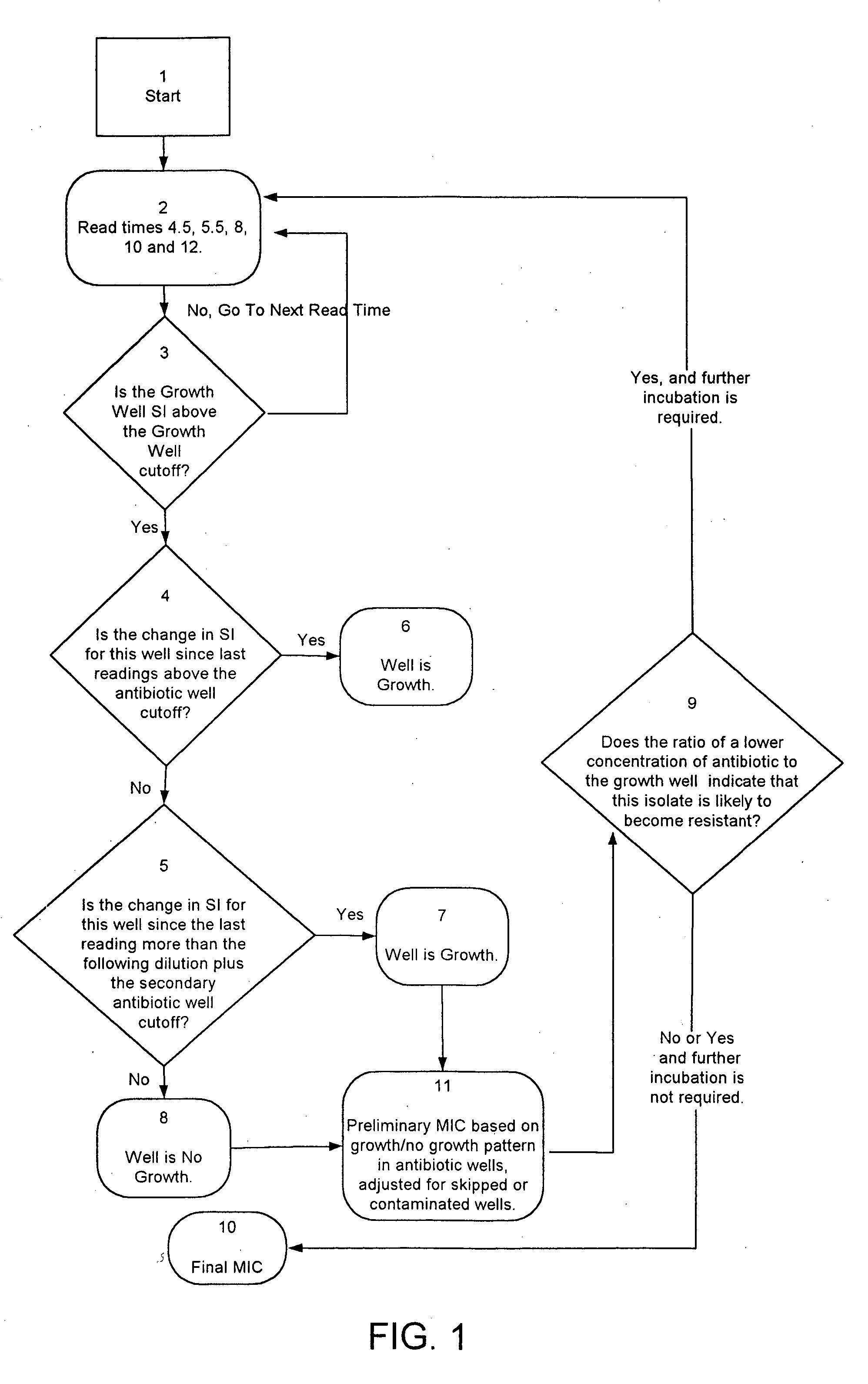
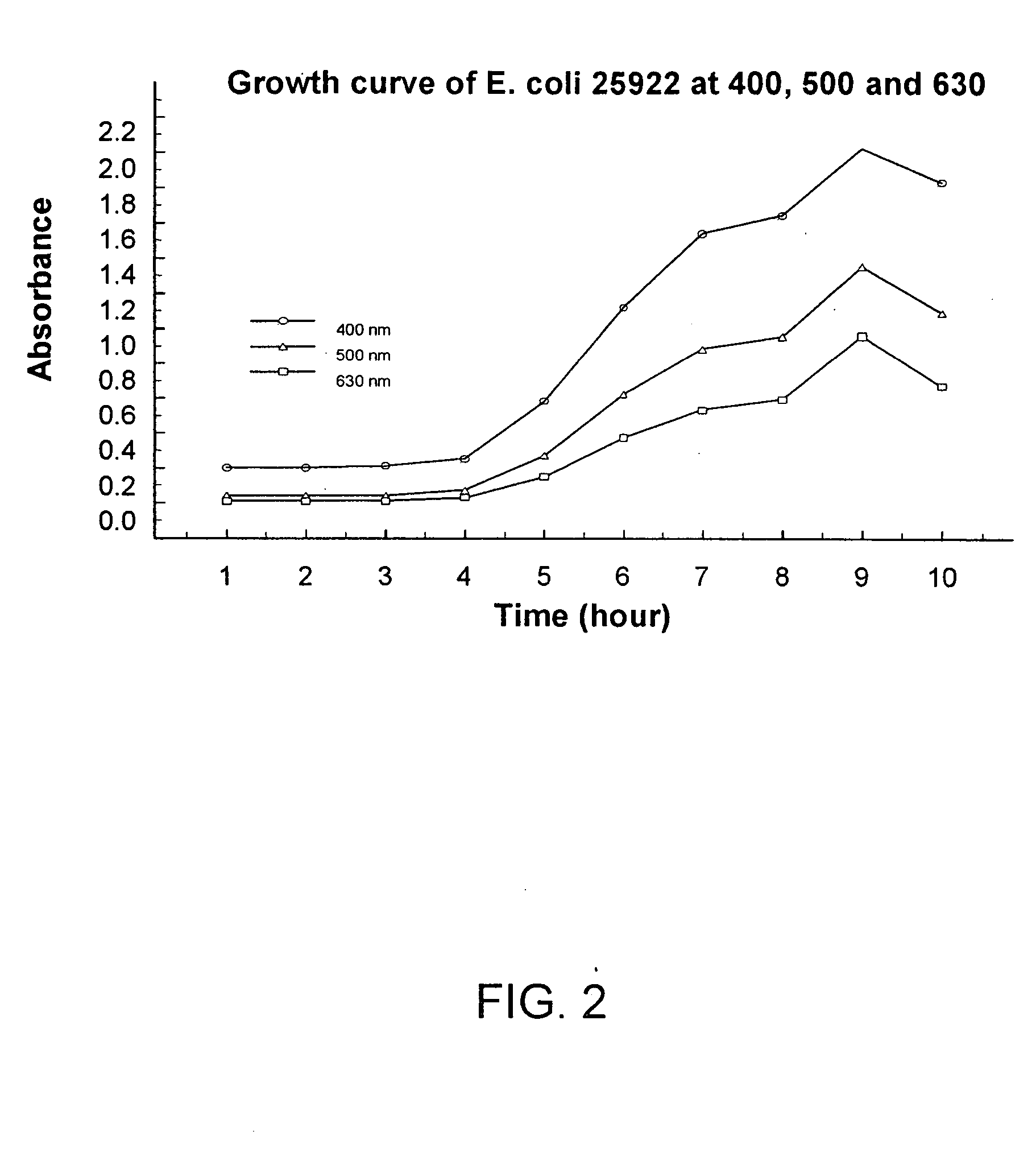
Combined rapid susceptibility assay and microorganism identification system
a technology of rapid susceptibility assay and identification system, which is applied in the field of combined antimicrobial susceptibility testing and microorganism identification platform, can solve the problems of life-threatening and debilitating systemic and localized microbial infections, add significantly to patient suffering, and mortality resulting from infectious agents remains particularly high, so as to improve the sensitivity and accuracy of assays and systems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Standard Turbidimetric Assay Protocol 1: Gram Positive Assays
[0128] Although the following procedure relates to the use OF MICROSCAN® Dried Gram Positive MIC / Combo and Dried Gram Positive Breakpoint Combo panels, it is intended to be exemplary and not limiting. The standard turbidimetric assay protocol described herein is summarized below for the purpose of example but may be viewed in greater detail in the “DADE® MICROSCAN® Dried Gram Positive Procedural Manual, “Dade International Inc., West Sacramento, Calif. 95691 (1997), the disclosures of which are incorporated by reference herein.
[0129] The antimicrobial susceptibility tests are dehydrated miniaturizations of the broth dilution susceptibility test. Various antimicrobial agents are diluted in Mueller-Hinton broth with calcium and magnesium or Mueller-Hinton broth with additional supplementation to concentrations bridging the range of clinical interest.
[0130] For other specific tests, the use of different broths may be appr...
example 2
Standard Turbidimetric Assay Protocol 2: Gram Negative Assays
[0157] Although the following procedure relates to the use of MICROSCAN® Dried Gram Negative MIC / Combo and Dried Gram Negative Breakpoint Combo panels, it is intended to be exemplary and not limiting. The standard turbidimetric assay protocol described herein is summarized below for the purpose of example but may be viewed in greater detail in the “DADE® MICROSCAN® Dried Gram Negative Procedural Manual,” Dade International, Inc., West Sacramento, Calif. (1996), the disclosures of which are incorporated by reference herein.
[0158] The antimicrobial susceptibility tests are dehydrated miniaturizations of the broth dilution susceptibility test. Various antimicrobial agents are diluted in Mueller-Hinton broth with calcium and magnesium or Mueller-Hinton broth with additional supplementation to concentrations bridging the range of clinical interest. Breakpoint Combo panels use concentrations equivalent to the categorical brea...
example 3
Fluorescent ID Procedure
[0182] Although the following procedure relates to the use of MICROSCAN® Rapid Panels, it is intended to be exemplary and not limiting. The standard fluorescent ID protocol described herein is summarized below for the purpose of example but may be viewed in greater detail in the “Dade MICROSCAN® Rapid Gram Positive Procedural Manual, “Dade Behring Inc., West Sacramento, Calif. (1998), the disclosures of which are incorporated by reference herein.
[0183] The antimicrobial susceptibility tests are dehydrated miniaturizations of the broth dilution susceptibility test. Various antimicrobial agents are serially diluted in autoclaved distilled water with fluorogenic compounds to concentrations bridging the range of clinical interest. Trimethoprim / Sulfamethoxazole contains thymidine phosphorylase to reduce thymidine levels in the medium.
[0184] Fluorogenic substrates or fluorometric indicators are used for the identification of Micrococcaceae, Streptococcaceae, Li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com