Methods for producing silicon nitride films and silicon oxynitride films by thermal chemical vapor deposition
a technology of silicon oxynitride and thermal chemical vapor deposition, which is applied in the direction of chemical vapor deposition coating, coating, plasma technique, etc., can solve the problems of poor thermal stability, poor step coverage, and low film density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
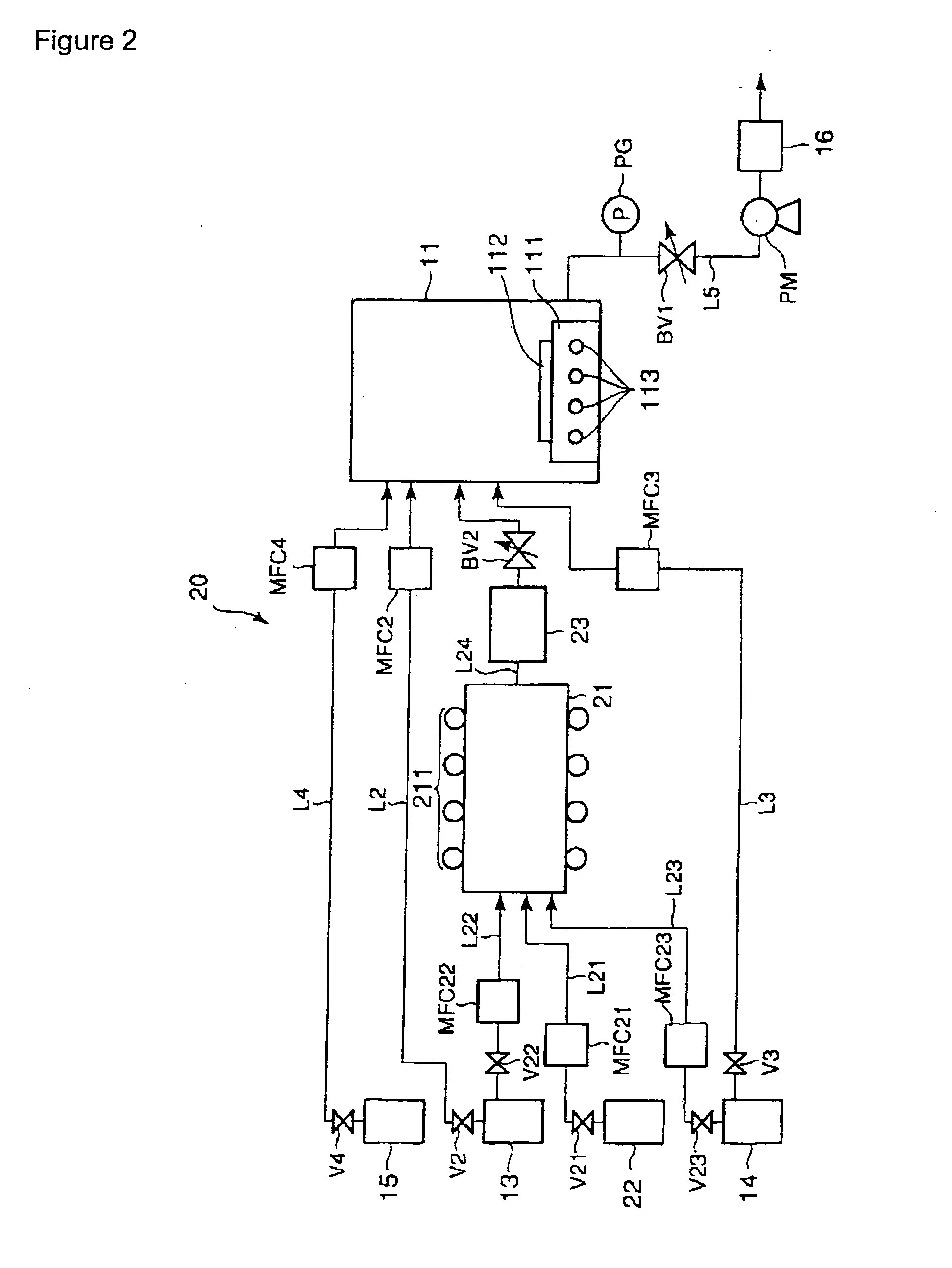
Image
Examples
example 1
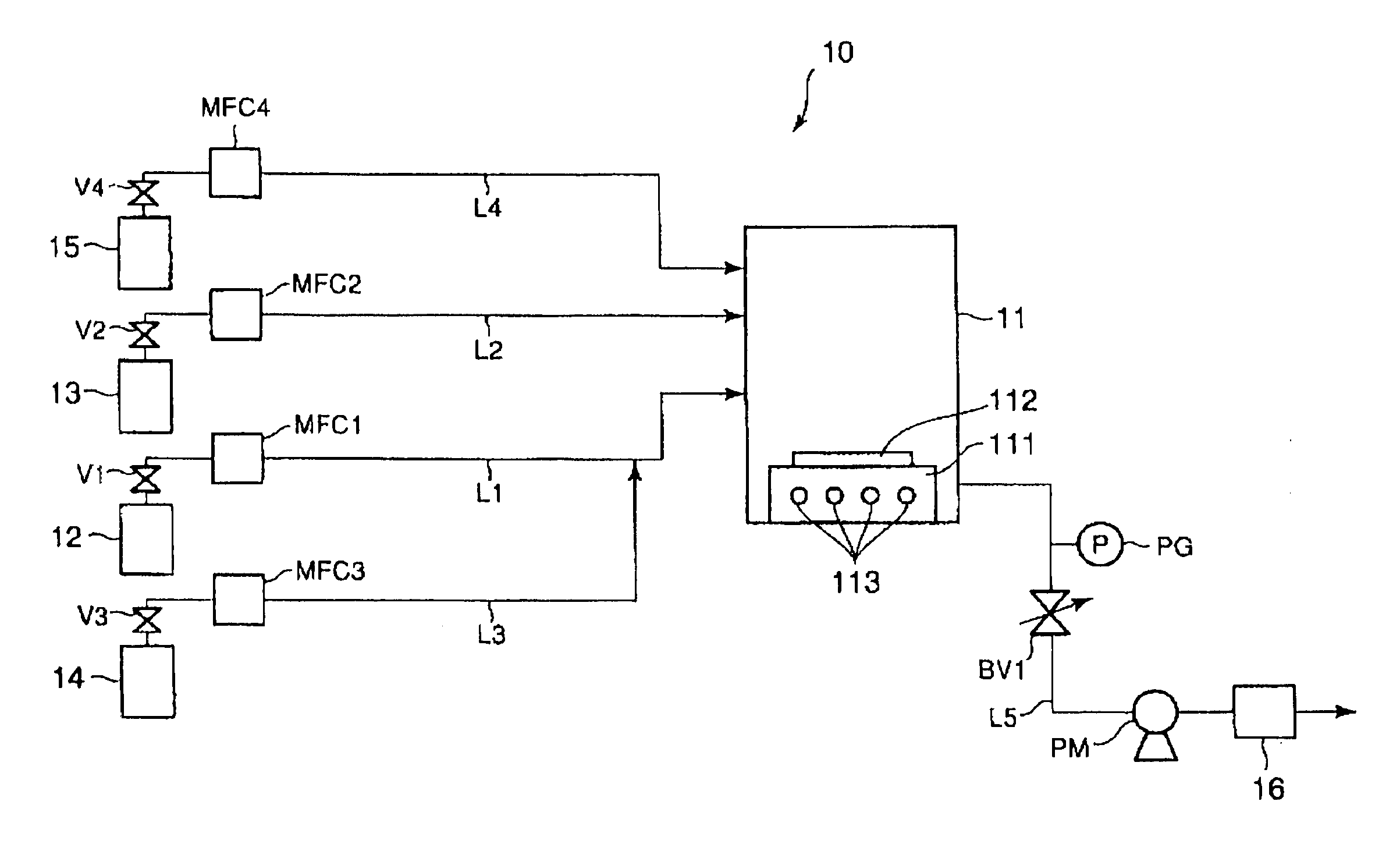
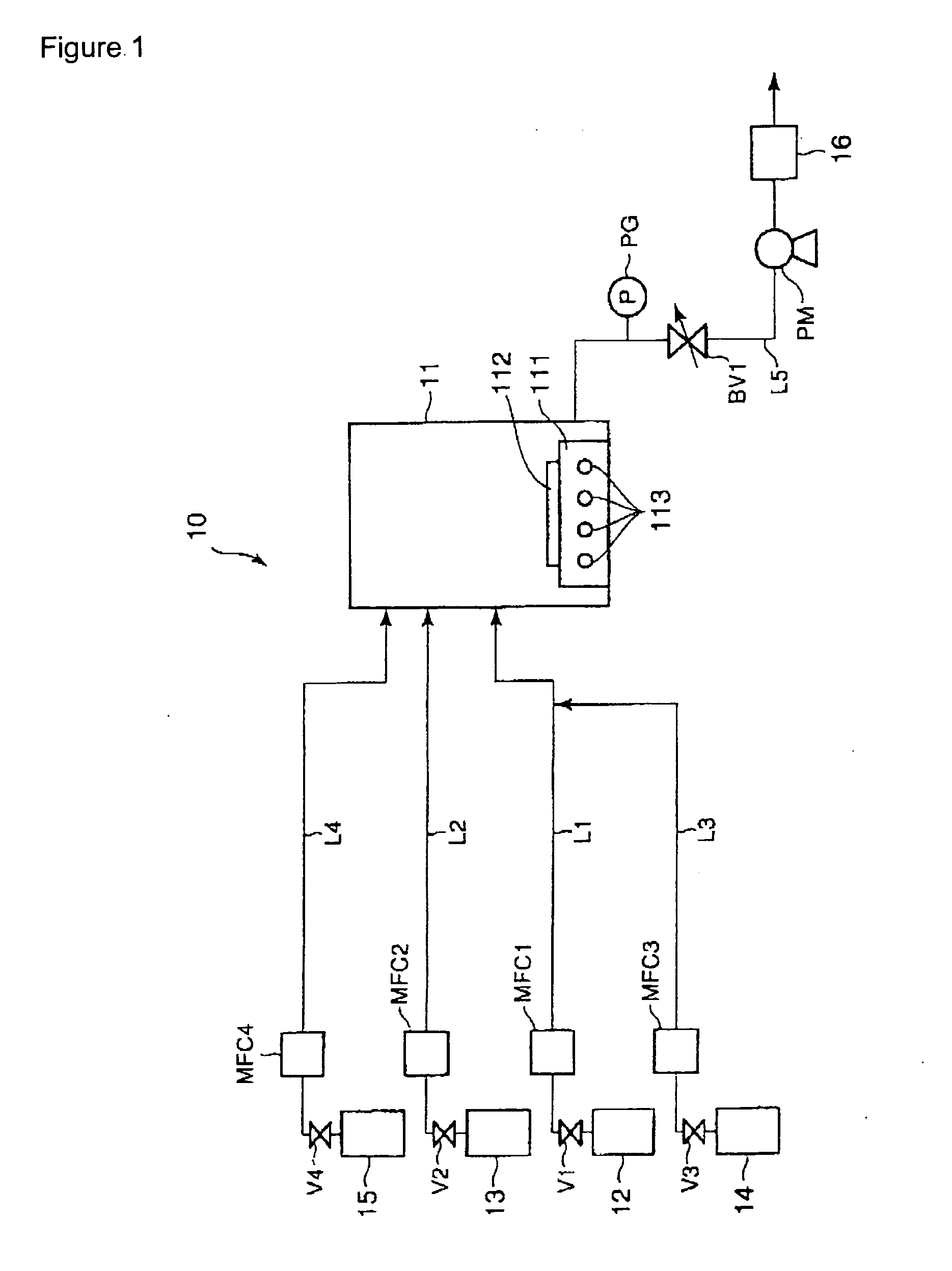
[0080] A production apparatus as illustrated in FIG. 1 (lacking, however, an oxygen source gas feed system) was used in this example. Ammonia gas and TSA gas were introduced into a CVD reaction chamber holding a silicon substrate and a silicon nitride film was formed on this silicon substrate under the following conditions. [0081] ammonia gas flow rate: 40 sccm [0082] TSA gas flow rate: 0.5 sccm [0083] pressure within the CVD reaction chamber: 1 torr [0084] reaction temperature: 640° C.
[0085] The resulting silicon nitride film was confirmed by Auger spectroscopy to have the composition Si0.81N. The deposition (growth) rate of this silicon nitride film was 17 Å / min. The NH3 / TSA flow rate ratio in this example was 80, and a stable film composition was achieved.
example 2
[0086] A production apparatus as illustrated in FIG. 1 (lacking, however, an oxygen source gas feed system) was used in this example. Ammonia gas and TSA gas were introduced into a CVD reaction chamber holding a silicon substrate and a silicon nitride film was formed on this silicon substrate under the following conditions. [0087] ammonia gas flow rate: 40 sccm [0088] TSA gas flow rate: 4 sccm [0089] pressure within the CVD reaction chamber: 1 torr [0090] reaction temperature: 560° C.
[0091] The resulting silicon nitride film was confirmed by Auger spectroscopy to have the composition Si1.04N. The deposition (growth) rate of this silicon nitride film was 6 Å / min. The NH3 / TSA flow rate ratio in this example was 10, and a stable film composition was achieved.
example 3
[0092] A production apparatus as illustrated in FIG. 1 (lacking, however, an oxygen source gas feed system) was used in this example. Ammonia gas and TSA gas were introduced into a CVD reaction chamber holding a silicon substrate, and a silicon nitride film was formed on this silicon substrate using a pressure within the CVD reaction chamber of 1 torr and a reaction temperature of 600° C. Silicon nitride film formation was carried out at different NH3 / TSA flow rate ratios over the range from 0 to 80. Auger spectroscopy was used to analyze the composition of the silicon nitride films obtained at the different NH3 / TSA flow rate ratios, and the compositional variation rate was calculated from the equation
compositional variation rate=−d(Si / N)dX
wherein X is the NH3 / TSA flow rate ratio. The results are reported in FIG. 3.
[0093] The results reported in FIG. 3 show that the film compositional variation rate due to changes in the flow rate ratio assumed very small values when the NH3 / TSA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| pressures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com