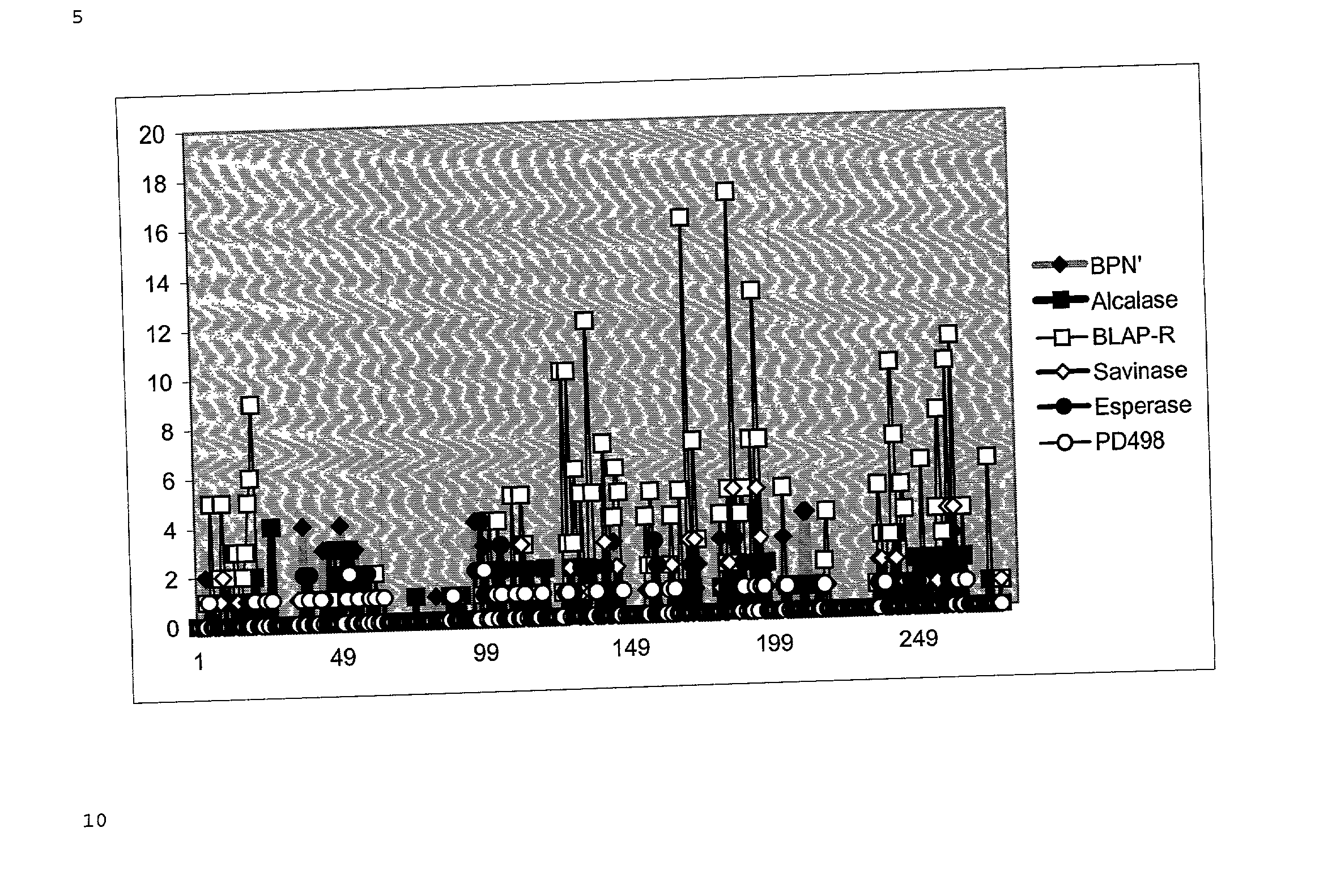
Protein variants having modified immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Epitope Sequences and Epitope Patterns
[0553] High diversity libraries (1012) of phages expressing random hexa-, nona- or dodecapetides as part of their membrane proteins, were screened for their capacity to bind purified specific rabbit lgG, and purified rat and mouse IgGl and IgE antibodies. The phage libraries were obtained according to prior art (se WO 9215679 hereby incorporated by reference).
[0554] The antibodies were raised in the respective animals by subcutaneous, intradermal, or intratracheal injection of relevant proteins (e.g. proteases, lipolytic enzymes, amylases, oxidoreductases) dissolved in phosphate buffered saline (PBS). The respective antibodies were purified from the serum of immunised animals by affinity chromatography using paramagnetic immunobeads (Dynal AS) loaded with pig anti-rabbit lgG, mouse anti-rat IgG1 or IgE, or rat anti-mouse IgGl or IgE antibodies.
[0555] The respective phage libraries were incubated with the IgG, IgG1 and IgE an...
example 2
Localisation of Epitope Sequences and Epitope Areas on the 3D-Structure of Acceptor Proteins
[0566] Epitope sequences were assessed manually on the screen on the 3D-structure of the protein of interest, using apropriate software (e.g. SwissProt Pdb Viewer, WebLite Viewer).
[0567] In a first step, the identified epitope patterns were fitted with the 3D-structure of the enzymes. A sequence of at least 3 amino acids, defining a specific epitope pattern, was localised on the 3D-structure of the acceptor protein. Conservative mutations (e.g. aspartate for glutamate, lysine for arginine, serine for threonine) were considered as one for those patterns for which phage display had evidenced such exchanges to occur. Among the possible sequences provided by the protein structure, only those were retained where the sequence matched a primary sequence, or where it matched a structural sequence of amino acids, where each amino acid was situated within a distance of 5 Å from the next one. Occasion...
example 3
Epitope Areas
[0573] It is common knowledge that amino acids that surround binding sequences can affect is binding of a ligand without participating actively in the binding process. Based on this knowledge, areas covered by amino acids with potential steric effects on the epitope-antibody interaction, were defined around the identified epitopes. Practically, all amino acids situated within 5 Å from the amino acids defining the epitope were included. The accessibility criterium was not included for defining epitope areas, as hidden amino acids can have an effect on the surrounding structures.
[0574] For Savinase, the following amino acid residues belong to the epitope area that correspond to each epitope sequence indicated in Table 2:
sav1.1A1Q2S3P5H39P40D41L42N43G63T66H67A69G70T71A73A74L75N77S78I79G80V81L82G83N204V205Q206S207T208Y209P210S212T213Y214A215S216L217sav1.2S153G154N155S156G157A158G160S161I162S163A169R170A174M175A176V177G178R186F189S190Q191Y192G193A194G195L196D197I198V199T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com