Antiallergic agent, utilization thereof for reducing allergy and method of reducing allergy
an antiallergic agent and allergy technology, applied in the field of antiallergic agents, can solve the problems of side effects, no fundamental therapeutic agent has been found for specifically reducing ige antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(Preparation of IgE-elevated Mice)
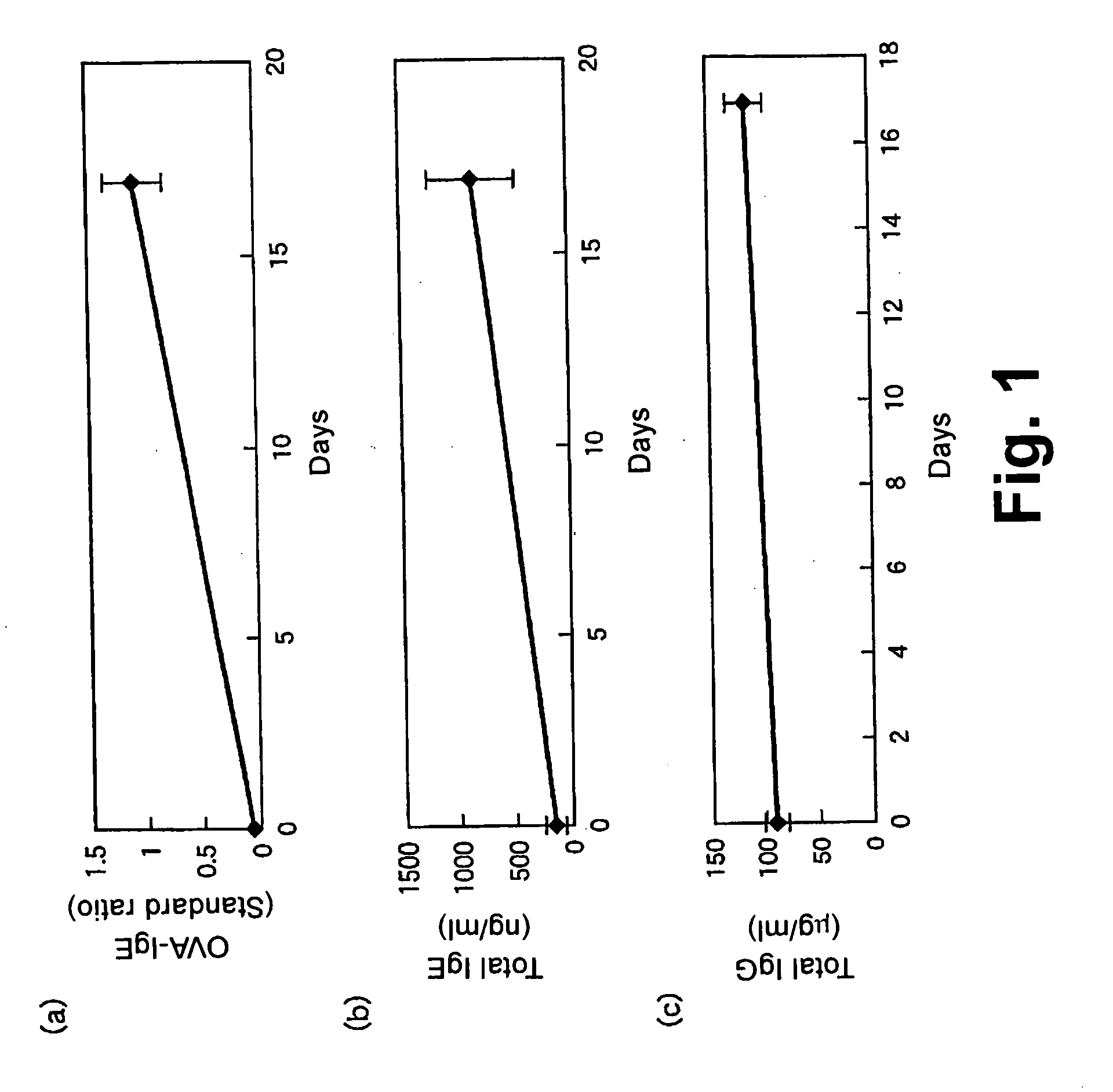
[0064] Male BALB / c mice were obtained from Charles River Japan, and raised under free access to CE-2 (CLEA Japan, Inc.) as a feed. 10 μg of ovalbumin (abbreviated as OVA hereinbelow, manufactured by SIGMA CHEMICAL CO.) and 2 mg of aluminum hydroxide (WAKO PURE CHEMICAL INDUSTRIES, LTD.) as an adjuvant were suspended in 300 μl of saline. Ten of the above mice at six weeks old were injected intraperitoneally with this suspension on the first day of sensitization and on day 4 for primary sensitization. For secondary sensitization, the nose of each mouse was soaked in an OVA antigen solution containing 25 mg OVA / ml of saline for three seconds, and this soaking operation was repeated three times as one cycle. Two cycles of soaking operation was performed per day, and the daily soaking was performed from day 10 to day 16 to prepare IgE-elevated mice.
[0065] Blood samples were obtained from the ophthalmic veins of the IgE-elevated mice on the first day a...
example 2
(Comparison in Effect of Various Lactic Acid Bacteria)
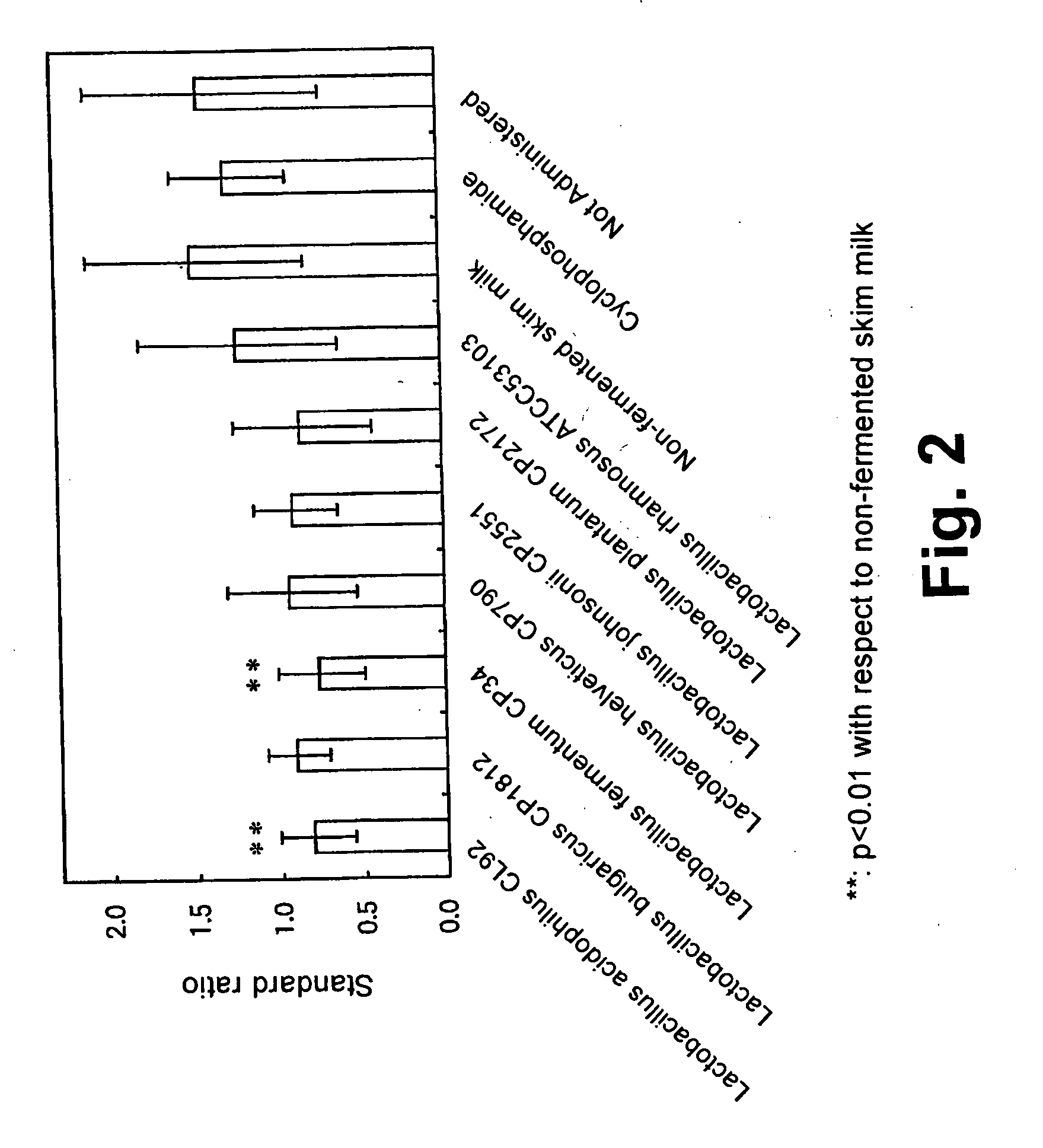
[0073] Each of the lactic acid bacterial strains shown in Table 1 was precultured in MRS medium overnight at 37° C., and the cells were harvested by centrifugation at 3000 rpm for 10 minutes. 9% (W / V) reconstituted skim milk (containing 0.1% (W / V) yeast extract (manufactured by DIFCO)) was fermented with the collected cells at 37° C. until the milk was coagulated. After the fermentation, the total cell count of each fermented milk was measured. The results are shown in Table 1.
TABLE 1Total cellcountStrain(cells / ml)Lactobacillus acidophilus CL92 (BP-4981)1.9 × 108Lactobacillus bulgaricus CP18121.5 × 108Lactobacillus fermentum CP345.3 × 108Lactobacillus helveticus CP7902.4 × 108Lactobacillus johnsonii CP25512.7 × 108Lactobacillus plantarum CP21725.9 × 108Lactobacillus rhamnosus ATCC531031.0 × 108
[0074] Next, IgE-elevated mice were prepared in the same way as in Example 1, and the blood OVA-IgE was measured on day 18 of sensitiz...
example 3
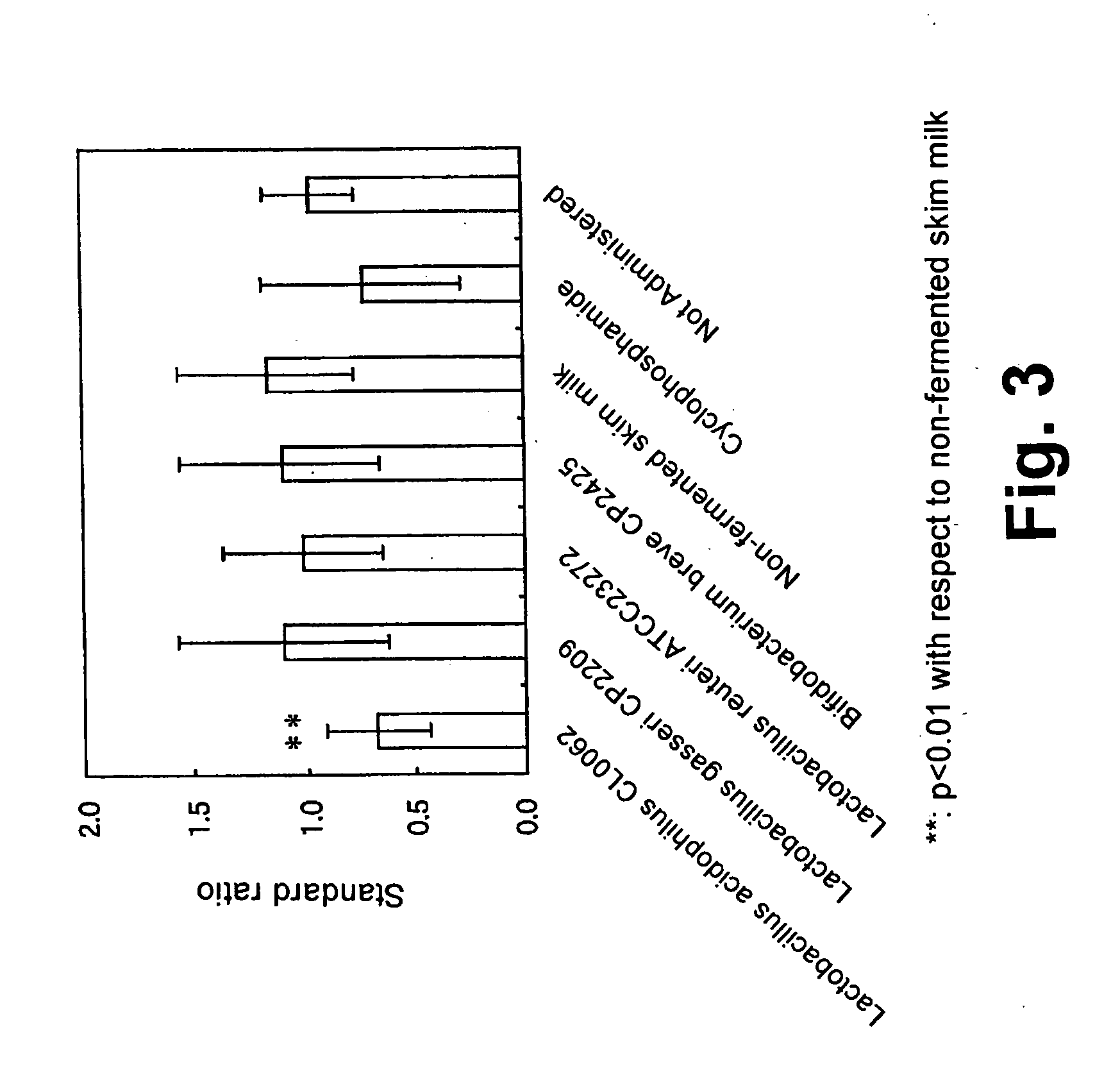
[0076] The procedure in Example 2 was followed except that the lactic acid bacterial strains shown in Table 2 were used. The results of measurement of the total cell count in each fermented milk are shown in Table 2. The results of measurement of the blood OVA-IgE are shown in FIG. 3.
TABLE 2Total cellcountStrain(cells / ml)Lactobacillus acidophilus CL0062 (BP-4980)4.40 × 108Lactobacillus gasseri CP22094.30 × 108Lactobacillus reuteri ATCC232729.60 × 108Bifidobacterium breve CP24251.30 × 108
[0077] As shown in FIG. 3, in the group of mice given Lactobacillus acidophilus fermented milk, significant inhibitory effect (p<0.01) in OVA-IgE level was observed, compared to the group given non-fermented skim milk. No significant difference was observed in total IgG level in blood (not shown).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com