Methods for the preparation of metallic alloy nanoparticles and compositions thereof
a technology of metal alloy nanoparticles and compositions, applied in the direction of magnetic materials, magnetic bodies, transportation and packaging, etc., can solve the problems of complex and demanding methods, contamination of reaction byproducts, contamination of mechanical parts, etc., and achieve the effect of easy production of specific chemical compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Metal Alloy Formation Using Cyanogels
[0047] A. Synthesis of Cyanogels: Bulk cyanogels were synthesized by the reaction of an aqueous solution of Na2PdCl4 (Pressure Chemical Co.) with the potassium or sodium salt of one of the following cyanometall ate complexes: (Co(CN)6)3−, (Fe(CN)6)3−, (Fe(CN)6)4−, (Fe(CN)5L)3−, (Cr(CN)6)3−, (Mn(CN)6)4−, (Ru(CN)6)3−, (Os(CN)6)3−, (Co(CN)6)3−, (Pt(CN)4)2−, (Pd(CN)4)2−, (Ni(CN)4)2−, (Mo(CN)8)4−, and (W(CN)8)4−. Cyanometallate complexes were purchased from Aldrich, except for the Mo and W complexes which were synthesized using procedures described in N H Furman and C O Miller, Inorganic Synthesis, Vol. 3, 160 (L F Audrieth, ed., McGraw-Hill, 1950). In all cases, equimolar (molarity ranging from 5 mM to 0.9 mM) aqueous solutions of Na2PdCl4 and the cyanometallate of interest were prepared, 20 ml of the chloropalladate solution was mixed with 10 ml of the cyanometallate solution, and the mixture was reacted at room temperature. After mixing the soluti...
example 2
Synthesis of 31 nm Pd / Co Alloy Nanoparticles
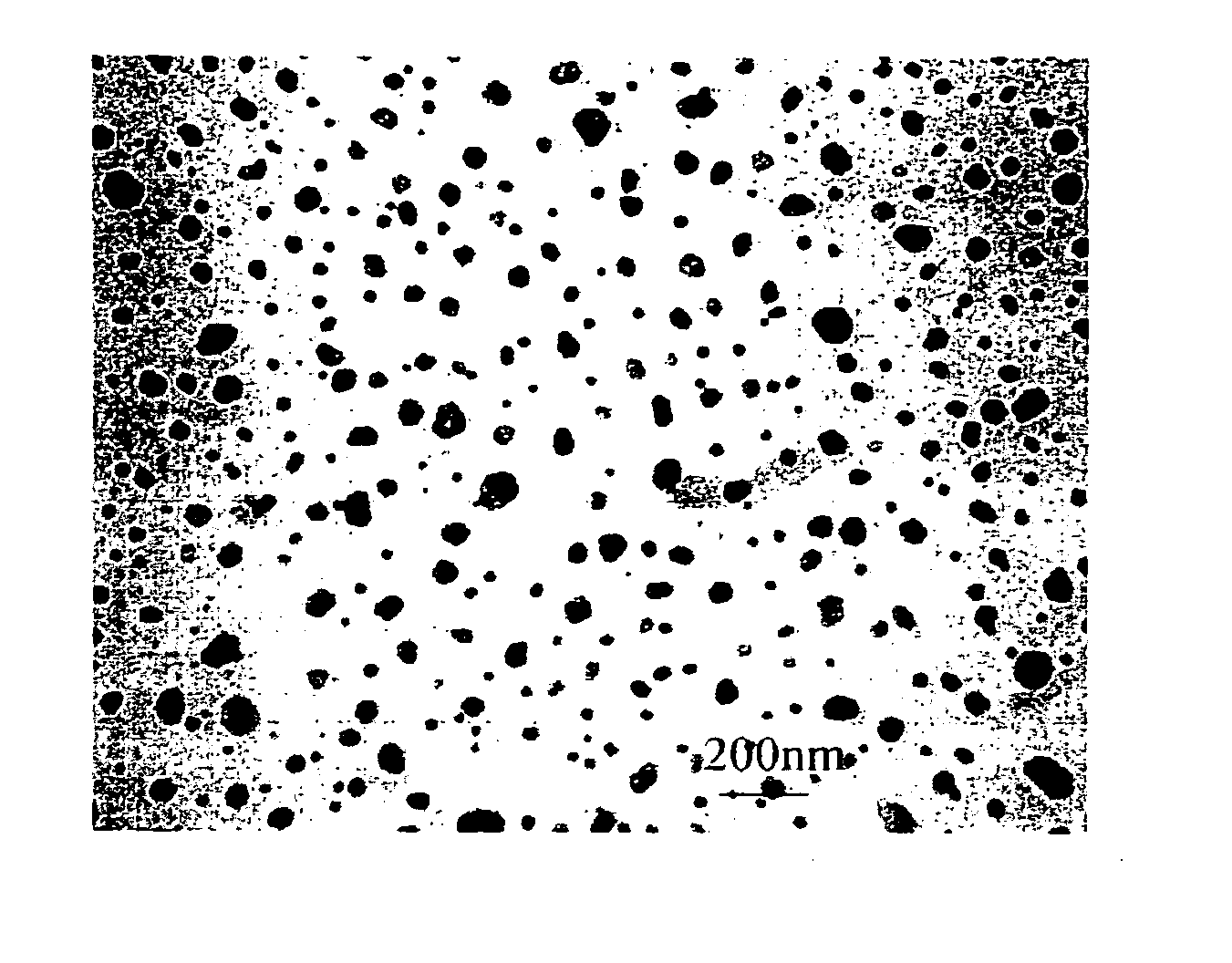
[0051] Aqueous solutions of 0.06 M Na2PdCl4 (Pressure Chemical Co.) and 0.06 M K3Co(CN)6 (Aldrich) were mixed at 3:1 ratio at ambient temperature. Immediately after mixing, the mixture was quickly spin-coated onto a substrate at 3000 rpm to form a thin film. A volume of 0.25 ml was coated over a 5 cm2 substrate by dosing the surface with 0.05 ml aliquots over a 10 s period. The film was allowed to dry at room temperature, and was then sintered at 650° C. under a flow of argon by placing the sample in a three zone furnace (Carbolite TZF 12 / 65 / 550) at room temperature and heating to 650° C. over a three hour period. The sample was then held at the sinter temperature for one hour and allowed to cool down to room temperature in the furnace (overnight) under a flow of argon. Pd / Co alloy particles having an average diameter of 31 nm±20 nm were formed.
[0052]FIG. 1 is the TEM image of the particles. TEM showed that the particles were non-agglome...
example 3
Synthesis of 18 nm Pd / Co Alloy Nanoparticles
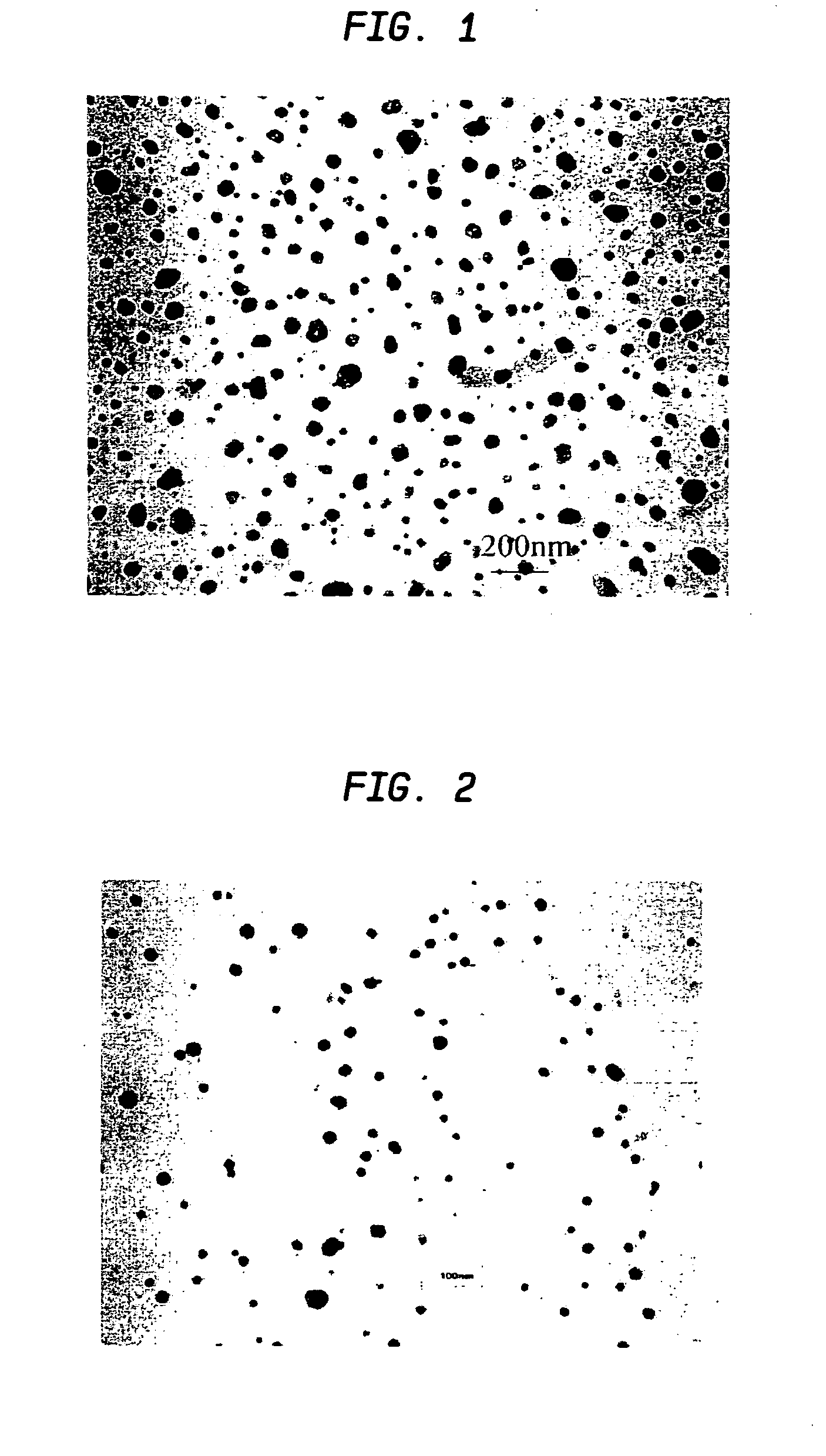
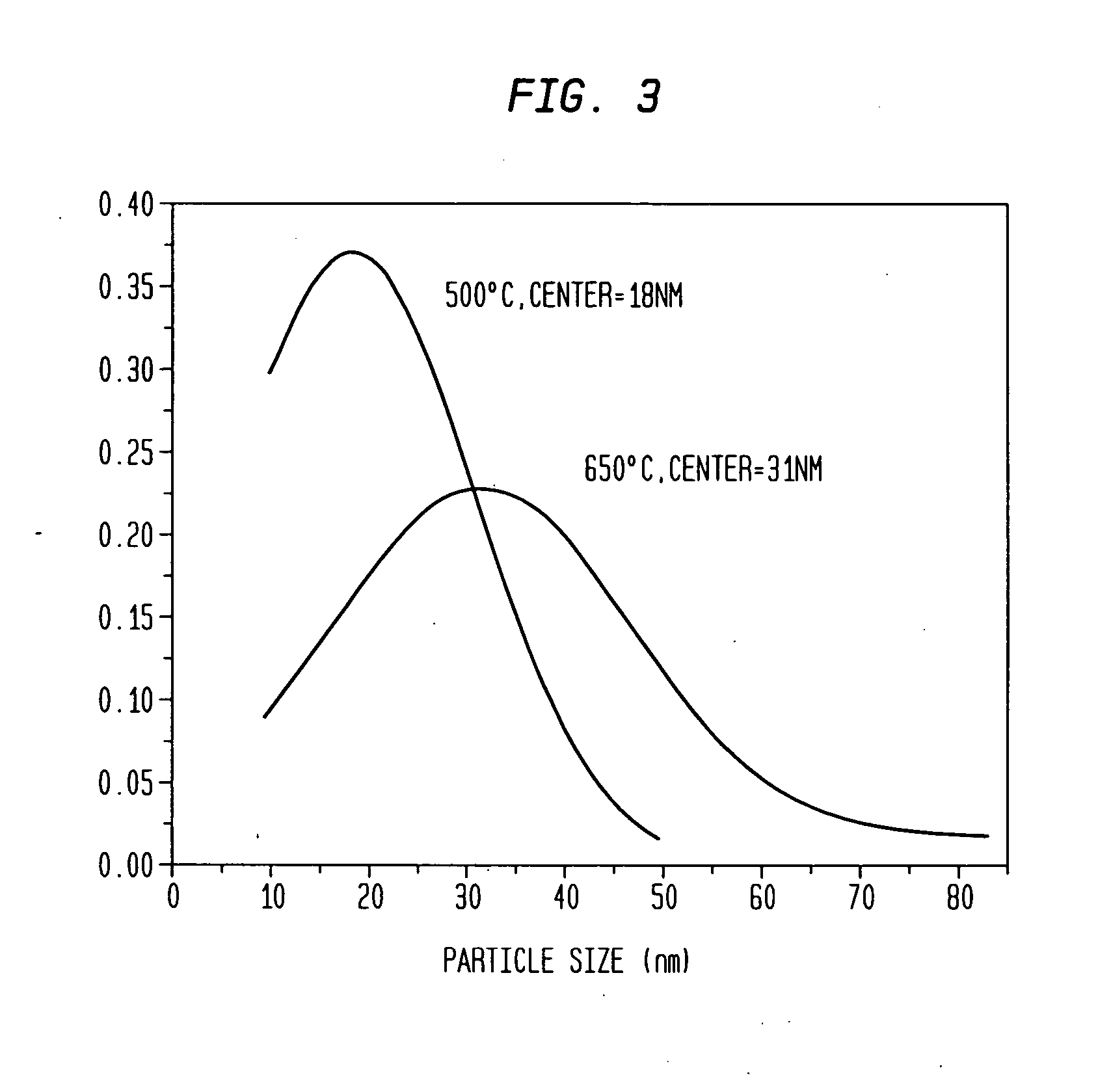
[0053] Aqueous solutions of 0.06 M Na2PdCl4 and 0.06 M K3Co(CN)6 were mixed at 3:1 ratio at ambient temperature. Immediately after mixing, the mixture was quickly spin-coated onto a substrate at 3000 rpm to form a thin film. A volume of 0.25 ml was coated over a 5 cm2 substrate by dosing the surface with 0.05 ml aliquots over a 10 s period. The film was allowed to dry at room temperature, and was then sintered at 500° C. under a flow of argon by placing the sample in a three zone furnace (Carbolite TZF 12 / 65 / 550) at room temperature and heating to 500° C. over a three hour period. The sample was then held at the sinter temperature for one hour and allowed to cool down to room temperature in the furnace (overnight) under a flow of argon. Polydispersed Pd / Co alloy particles having an average diameter of 18 nm±14 nm were formed (see TEM analysis in FIG. 2). The particle size distribution is shown in FIG. 3. Electron microprobe analysis of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com