Anti-oxidant macromonomers and polymers and methods of making and using the same
a macromonomer and polymer technology, applied in the field of anti-oxidant macromonomers and polymers and methods of making and using the same, can solve problems such as inefficiency of methods, and achieve the effects of less prone to oxidation, and short-term and long-term stability towards oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
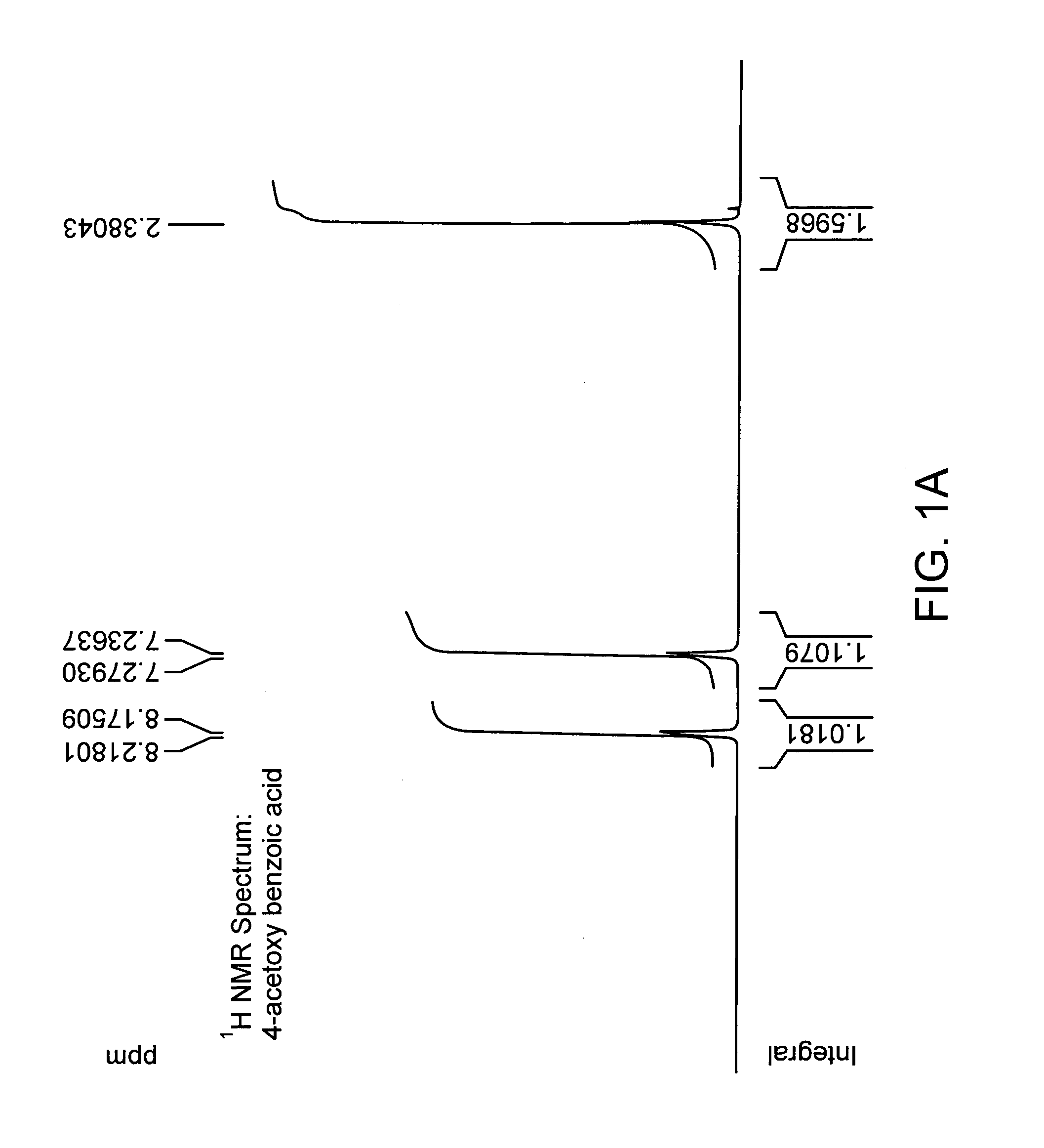
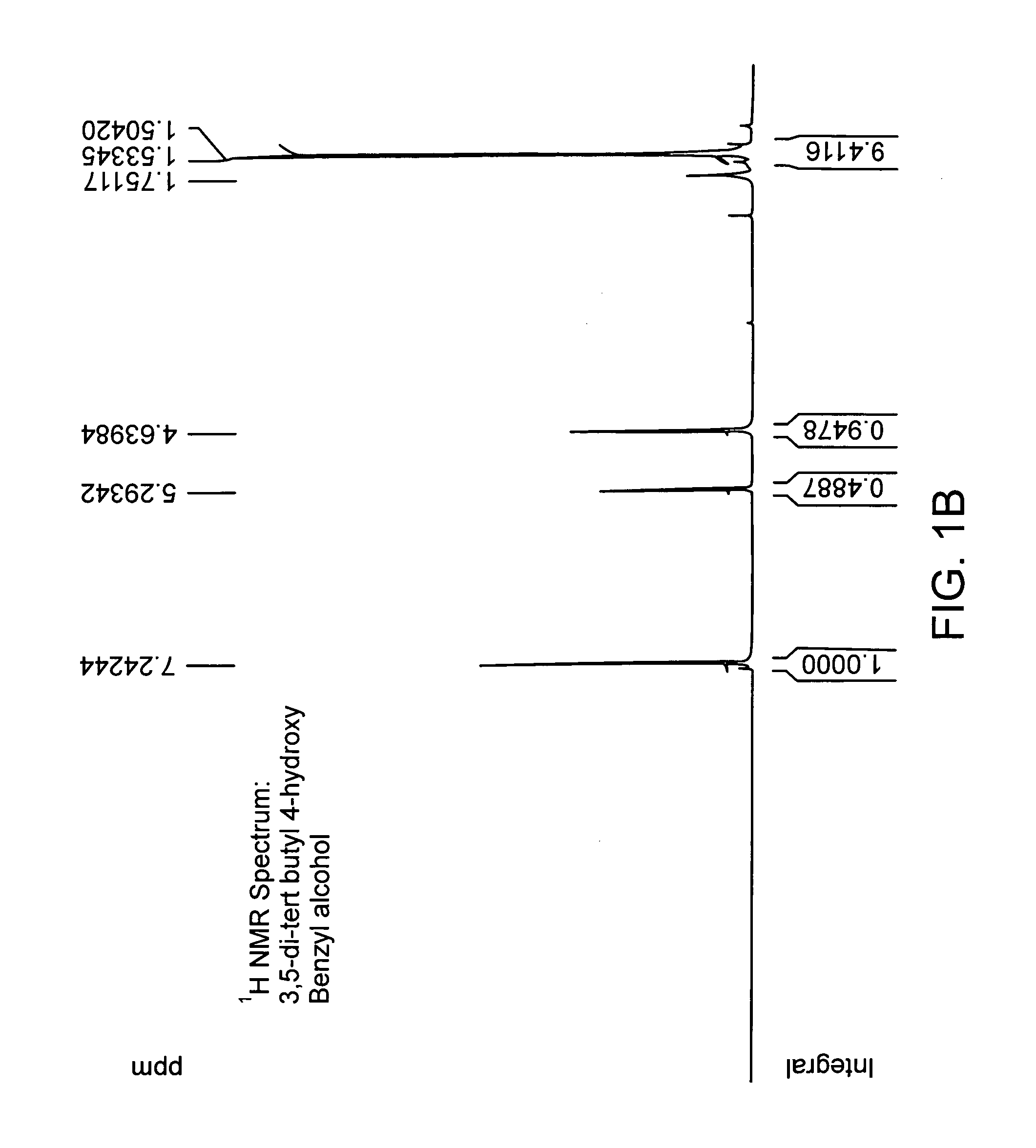
[0194] Chemical coupling of acid chloride and antioxidant-alcohol. Thionyl chloride was added drop wise to the suspension of 4-acetoxy benzoic acid in chloroform and the reaction mixture was refluxed. After refluxing the reaction mixture for 4 hours; chloroform and excess thionyl chloride were distilled out under vacuum. The white colored acid chloride product was dried under vacuum for 2 hours and then dissolved in dry dichloromethane. The solution of triethylamine and 3, 5 di-tert-butyl-4-hydroxy-benzyl alcohol in dry dichloromethane was added drop wise to it to obtain a yellow colored clear solution and the reaction mixture was stirred for additional 5 hours at room temperature in nitrogen atmosphere. The saturated aqueous sodium bicarbonate solution was then added and the reaction mixture was stirred for additional 30 minutes. The organic layer was separated and triethylamine-hydrochloride was washed off with water, and the product was dried and evaporated under vacuum and subje...
example 2
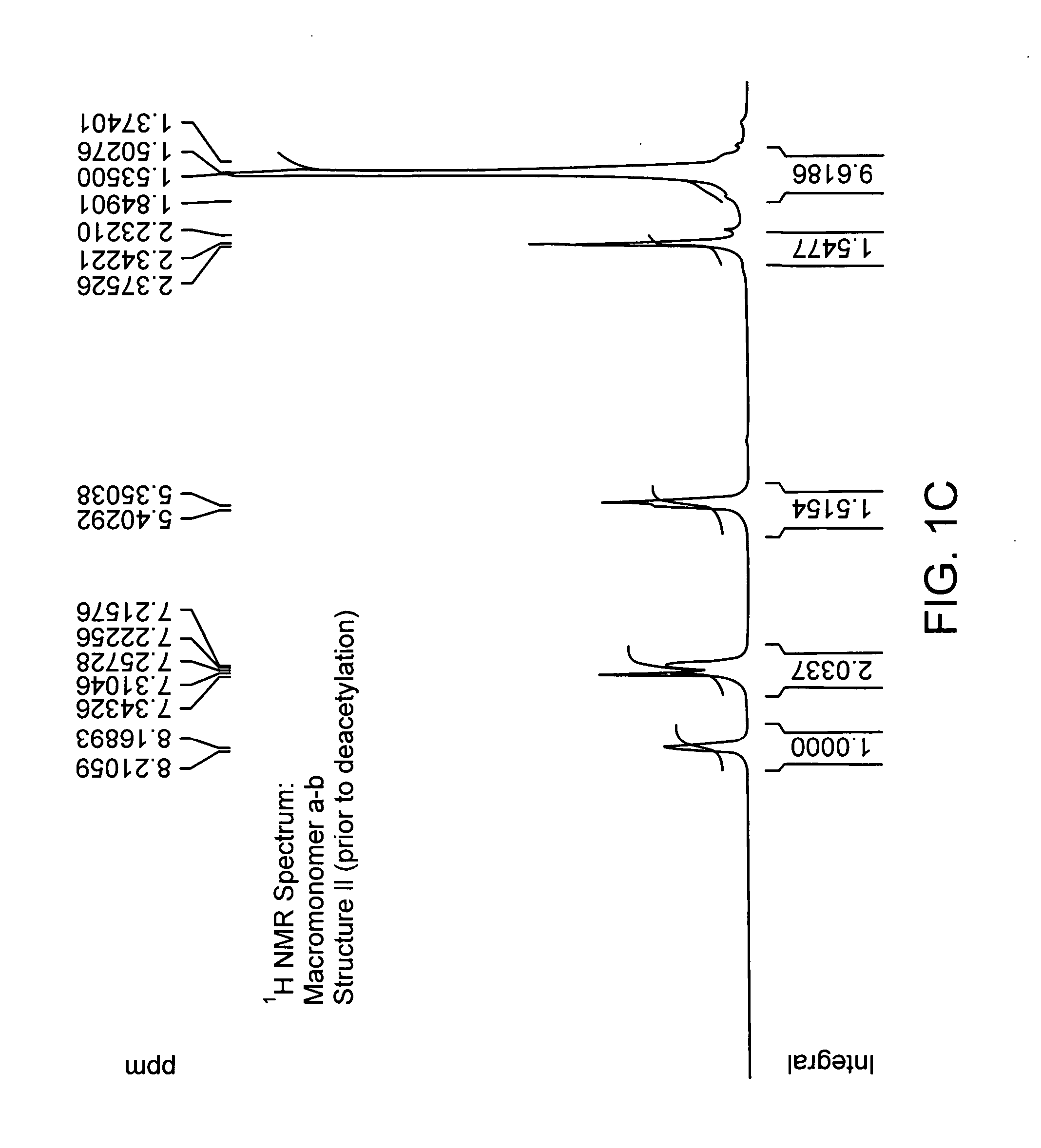
[0196] Enzymatic synthesis of antioxidant macromonomer, 4-hydroxy phenyl acetic acid-3,5-di-tert butyl 4-hydroxybenzyl alcohol ester. To the suspension of 3, 5 di-tert-butyl-4-hydroxy-benzyl alcohol and 4-hydroxy-phenyl-acetic acid in toluene in the presence of molecular sieves was added Candida Antarctica LipaseB (novozyme 435). The reaction mixture was stirred at 60° C. for 20 hours. After the completion of reaction; macromonomer (Compound 5) was purified using column chromatography (ethyl acetate petroleum ether). The molecular structure of this compound was confirmed to Structure VII by high resolution proton NMR.
example 3
[0197] Enzymatic synthesis of antioxidant macromonomer, 4-hydroxy phenyl acetic acid-3,5-di-tert butyl 4-hydroxybenzyl alcohol ester involving transesterification. To the suspension of 3, 5 di-tert-butyl-4-hydroxy-benzyl alcohol and 4-hyroxy-phenyl-acetic acid methyl ester in toluene was added Candida Antarctica Lipase B(novozyme 435). The molecular sieves were added to trap methanol that was produced as a result of transesterification. The reaction mixture was stirred at 60° C. for 20 hours. Macromonomer compound (Compound 5) was separated using column chromatography. The formation of the compound was confirmed by high resolution proton NMR.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| OIT | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com