Pharmaceutical compositions containing venlafaxine
a technology of venlafaxine and venlafaxine, which is applied in the field of formulation and method of delayed burst release of venlafaxine, can solve the problems of rapid increase in blood plasma levels of active compounds, increased dosage, and increased dosage, and achieves enhanced bioavailability of venlafaxine. , the effect of enhancing the bioavailability of venlafaxin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-10
[0089] These examples are of illustrative implementations of the formulation according to the present invention with venlafaxine. It should be noted that all examples given herein use venlafaxine hydrochloride, referred to herein as “venlafaxine” for the purpose of brevity and without any intention of being limiting. The formulations were tested in vitro to determine the release profile, as described in greater detail below.
TABLE 1Different formulations of the corecontaining VLF, and CaP-containing TCDS coating560-8560-11560-15Tablets contentMg / tab%mg / tab%mg / tab%Core:Venlafaxine8526.88526.68526.6Crospovidone41.513.041.312.93912.2Ca Pectinate4714.7Microcrystalline16652.2169.352.912840celluloseEthylcellulosePolyvinylpyrrolidone15.9516.05.0134.0Colloidal Silicon6.01.96.42.061.9DioxideMagnesium Stearate3.11.02.00.620.6Total weight317.5100320100320100uncoated tabletCaP Coating:560-9560-12560-16CaP Ethylcellulose47.6%Cetyl Alcohol4.8%CaP47.6%
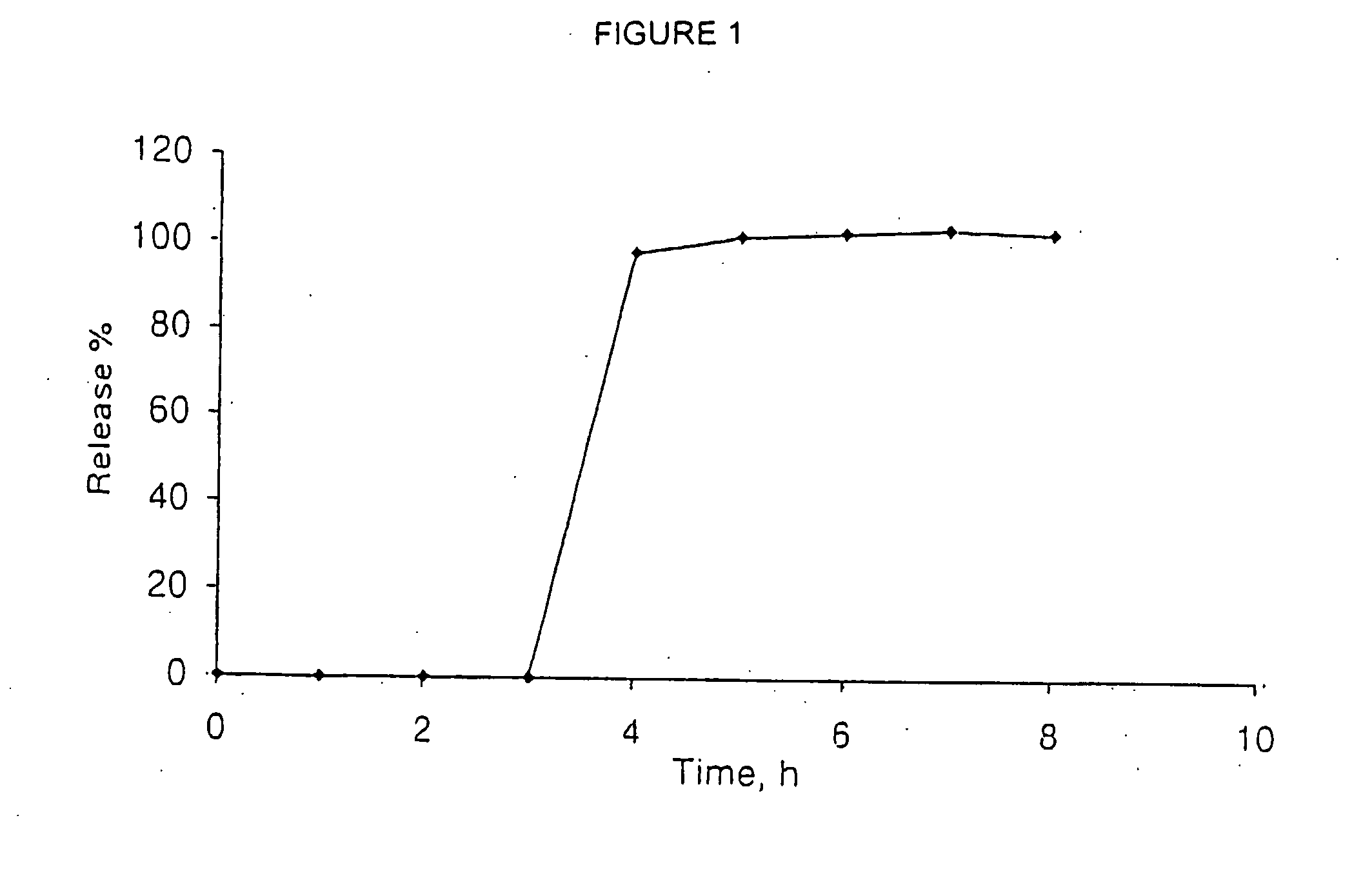
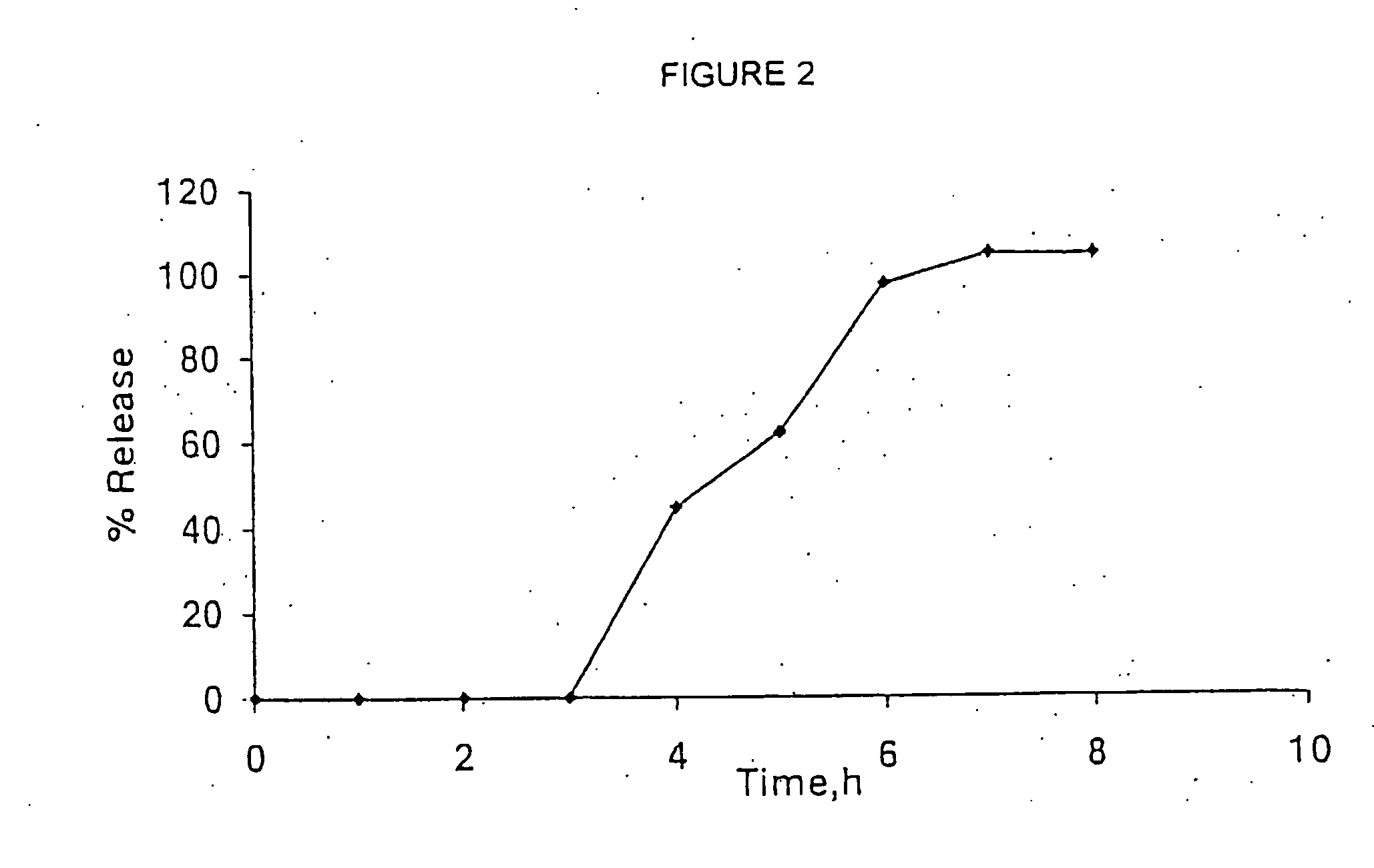
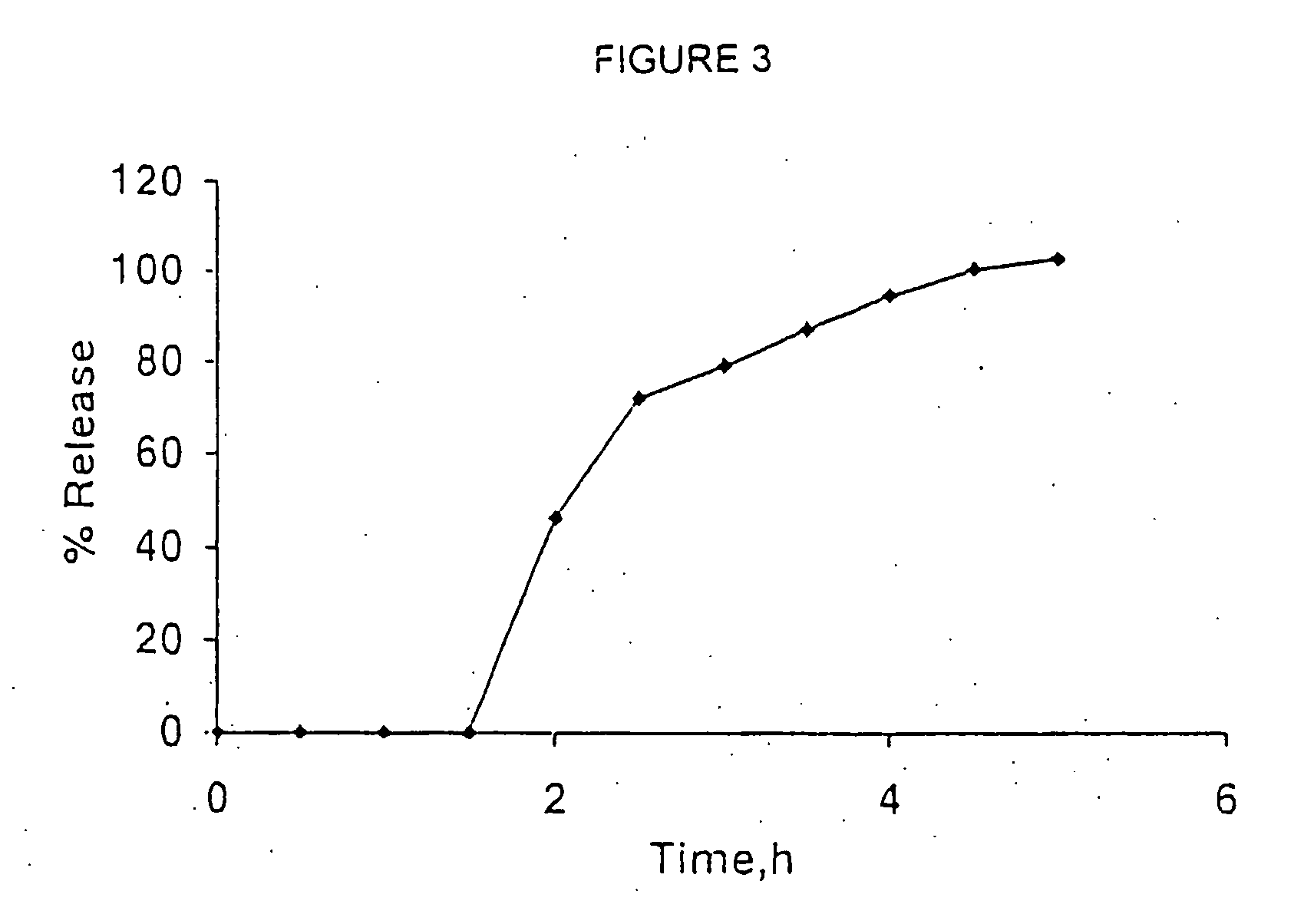
[0090] The in vitro release of venlafaxine fr...
example 1 (
560-9)
[0091] The cores were manufactured by dry mixing. Venlafaxine HCl (34 g) was mixed with colloidal silicon dioxide (2.4 g). The obtained mixture was sieved by sieve 600 microns and blended with crospovidone (16.6 g), microcrystalline cellulose (66.4 g) and polyvinyl pyrrolidone (6 g). Magnesium stearate (0.12 g) was passed through mechanical sieve equipped with 600 micron screen into the mixture and blended.
Tabletting
[0092] The tablets' blend was compressed with WICK single punch tabletting press equipped with suitable punches for providing sufficient active material and hardness sufficient for subsequent coating.
TCDS Coating
[0093] The formed cores were then coated with a TCDS coating containing calcium pectinate. (Ingredients and concentrations given in the tables above.)
[0094] The coating process was prepared and performed as follows. A weighed quantity of ethyl cellulose 20 (0.30 kg) was dissolved in ethanol (6.06 kg) to obtain clear solution, to which a weighed quant...
example 2 (
560-11)
[0097] Venlafaxine HCl(50 g) was mixed with disintegrator (cross-linked polyvinyl pyrrolidone-2.5 g) and binder-polyvinyl pyrrolidone and the granulation solution (water purified) was added. The blend was mixed until sufficient consistency was achieved. The granulated blend was dried.
[0098] The dried granulation blend was milled to obtain the desired particle size distribution of the final granulation blend.
[0099] Next, the process of blending was performed for the second part of the core. Colloidal silicon dioxide (3.2 g) was mixed with an additional amount of crospovidone (18.5 g) and sieved by a mechanical sieve equipped with a 600 micron screen into the previously obtained granulation blend. The obtained mixture was blended. Microcrystalline cellulose (84.7 g) was added into the mixture and the entirety was blended.
[0100] Magnesium stearate (1.0), which serves as lubricant, was passed through a mechanical sieve equipped with a 600 micron screen into the mixture and ble...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com