Optical detection of label-free biomolecular interactions using microreplicated plastic sensor elements
a technology of plastic sensor elements and optical detection, which is applied in the field of optical detection of label-free biomolecular interactions using micro-replicated plastic sensor elements, can solve the problems that methods have yet to yield commercially available high-throughput instruments, and achieve the effect of inexpensive incorporation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fabrication of a SWS Biosensor
[0216] An example of biosensor fabrication begins with a flat glass substrate that is coated with a thin layer (180 nm) of silicon nitride by plasma-enhanced chemical vapor deposition (PECVD).
[0217] The desired structure is first produced in photoresist by coherently exposing a thin photoresist film to three laser beams, as described in previously (Cowen, “The recording and large scale replication of crossed holographic grating arrays using multiple beam interferometry,” in International Conference on the Application, Theory, and Fabrication of Periodic Structures, Diffraction Gratings, and Moire Phenomena II, J. M. Lerner, ed., Proc. Soc. Photo-Opt. Instrum. Eng., 503, 120-129, 1984; Cowen, “Holographic honeycomb microlens,”Opt. Eng. 24, 796-802 (1985); Cowen & Slafer, “The recording and replication of holographic micropatterns for the ordering of photographic emulsion grains in film systems,”J. Imaging Sci. 31, 100-107, 1987. The nonlinear etching ...
example 2
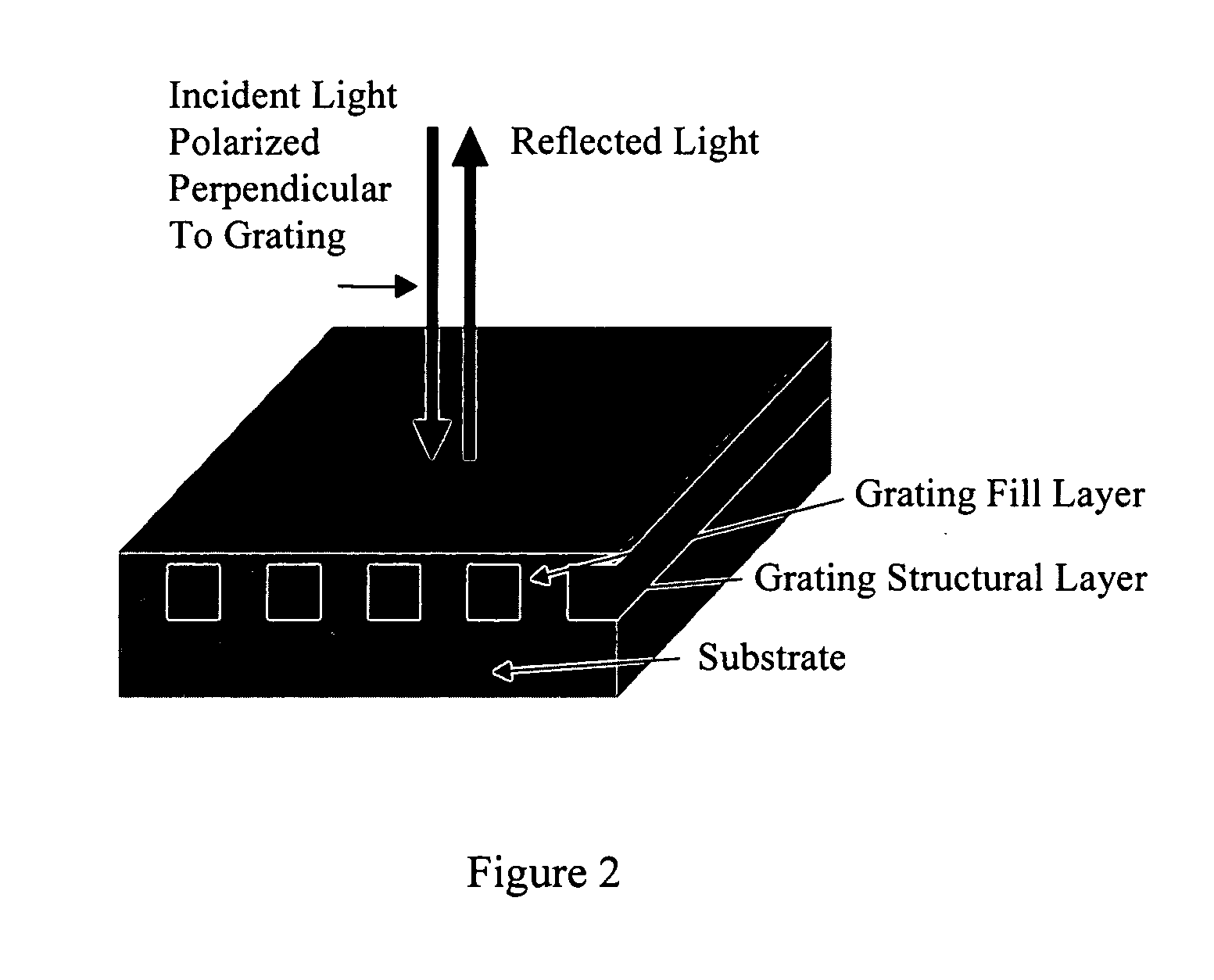
[0218] A SRVD biosensor was prepared by making five circular diffuse grating holograms by stamping a metal master plate into vinyl. The circular holograms were cut out and glued to glass slides. The slides were coated with 1000 angstroms of aluminum. In air, the resonant wavelength of the grating is ˜380 nm, and therefore, no reflected color is visible. When the grating is covered with water, a light blue reflection is observed. Reflected wavelength shifts are observable and measurable while the grating is covered with a liquid, or if a specific binding substances and / or binding partners cover the structure.
[0219] Both proteins and bacteria were immobilized onto the surface of a SRVD biosensor at high concentration and the wavelength shift was measured. For each material, a 20 μl droplet is placed onto a biosensor distinct location and allowed to dry in air. At 1 μg / ml protein concentration, a 20 μl droplet spreads out to cover a 1 cm diameter circle and deposits about 2×10−8 grams...
example 3
Computer Model of Biosensor
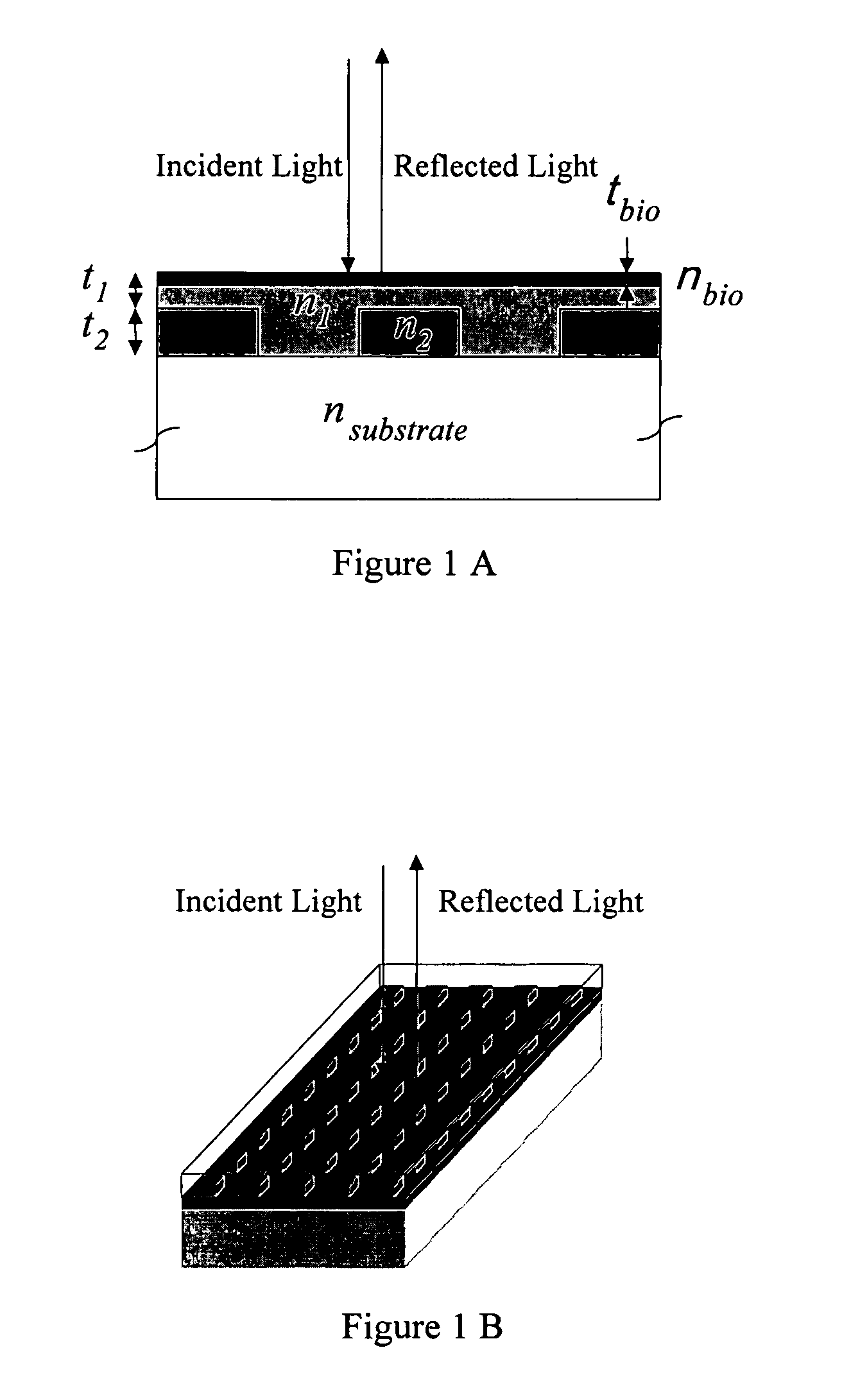
[0227] To demonstrate the concept that a resonant grating structure can be used as a biosensor by measuring the reflected wavelength shift that is induced when biological material is adsorbed onto its surface, the structure shown in FIG. 1 was modeled by computer. For purposes of demonstration, the substrate chosen was glass (nsubstrate=1.50). The grating is an optical pattern of silicon nitride squares (t2=180 nm, n2=2.01, k2=0.001) with a period of 510 nm, and a filling factor of 56.2% (i.e. 56.2% of the surface is covered with silicon nitride squares while the rest is the area between the squares). The areas between silicon nitride squares are filled with a lower refractive index material. The same material also covers the squares and provides a uniformly flat upper surface. For this simulation, a glass layer was selected (n1=1.40) that covers the silicon nitride squares by t2=100 nm. To observe the effect on the reflected wavelength of this structure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com