Refolding of membrane proteins
a membrane protein and protein technology, applied in the field of refolding of membrane proteins, can solve the problems of low expression rate, high system cost, and inactive protein production, and achieve the effect of improving the yield of native protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of an Expression Vector with cDNA for Receptor Protein
[0050] DNA sequences for several receptor proteins and also membrane proteins are in the EMBL database, in most cases, they do not have introns. With the help of primers, the required DNA can be produced via PCR from genomic DNA or via RT-PCR from mRNA.
[0051] This DNA is then cloned into an expression vector, which was constructed for the expression of a fusion protein. The carrier protein can be e.g. glutathione-S-transferase (GST), as described in the article by Kiefer et al. mentioned above, wherein a fusion protein was produced from the receptor OR5 and GST. The expression vector is transformed into a cell line which expresses the fusion protein. The protein is, in this procedure, not incorporated into the membrane, but exists at least partly aggregated in form of inclusion bodies in cytoplasm and is, thus, not correctly folded.
example 2
Isolation of Expressed Protein
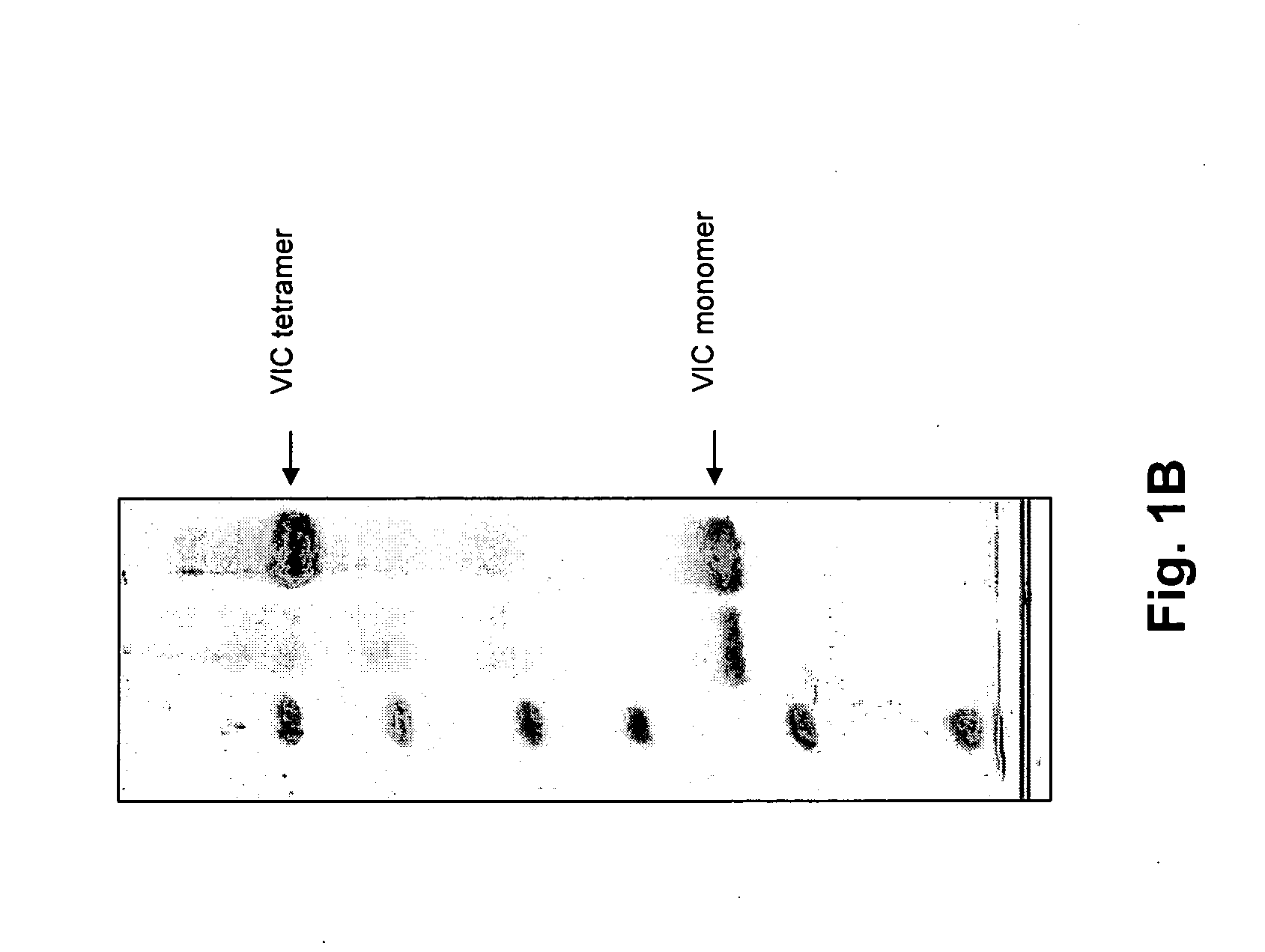
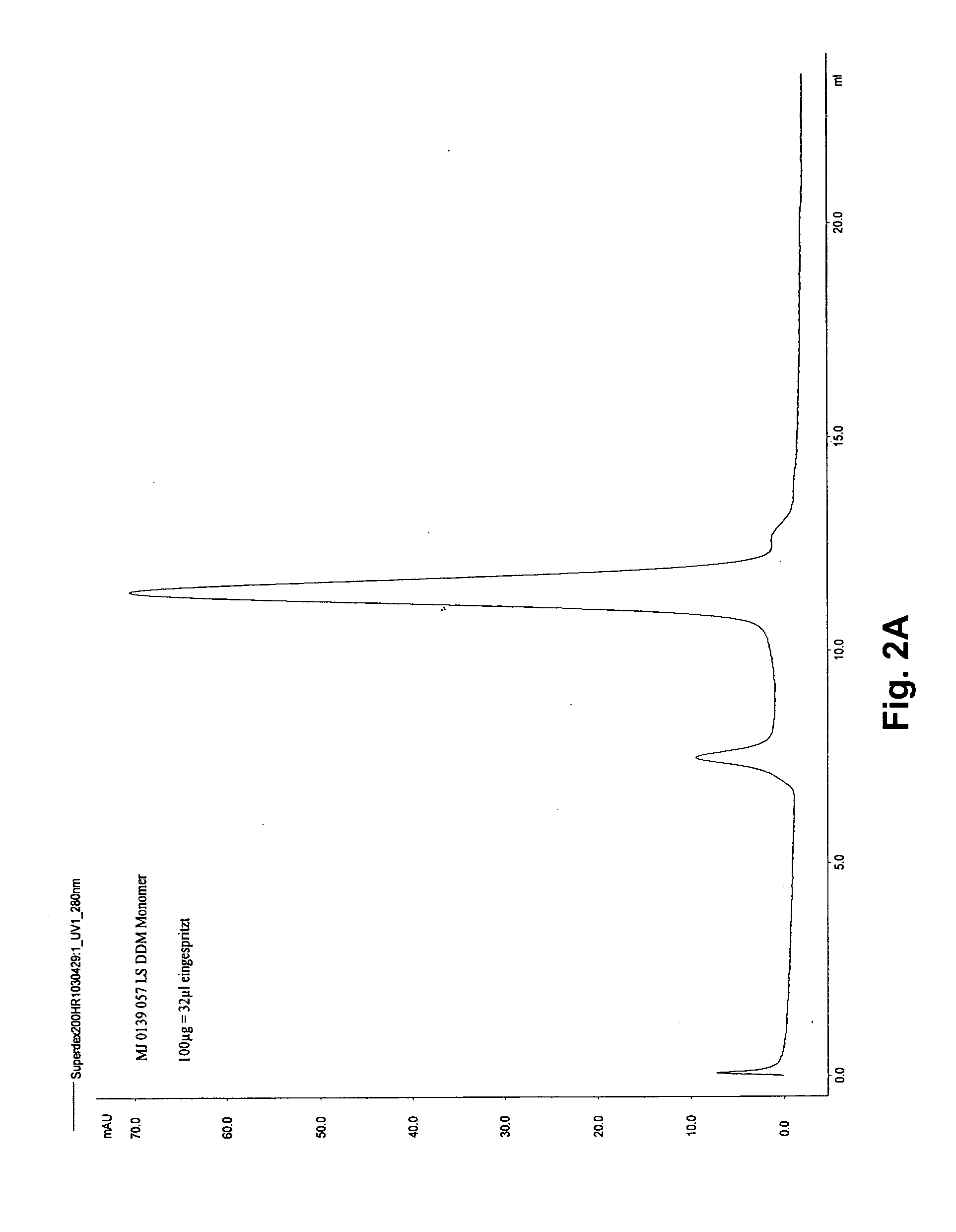
[0052] The cDNA of one of the following receptors is, in-frame, inserted into the vector pGEX2a-c-His: A0 adenosine receptor from the shark Squalus acanthias, human beta-2-adrenergic receptor, human neuropeptide YY1 receptor, human neuropeptide YY2 receptor, human melanocortine-1 receptor, human oxytocin receptor, tetrameric voltage gated ion channel from Salmonella typhimurium (Vic), tetrameric MJ ion channel from Methanococcus jannaschii (MJ). This vector contains downstream of the Tac-promotor the sequence encoding glutathione-S-transferase and a subsequent thrombin cleavage site, followed by a polylinker sequence and, finally, six histidine codons and a stop codon.
[0053] The vectors are transformed into E. coli, e.g. strains BL21 or BLR. The protein expression is induced by adding IPTG, and the cells are harvested after further three hours. After lysozyme treatment and homogenization by sonication, the membranes and inclusion bodies are separated ...
example 3
Solubization of the Protein
3.1 Voltage Gated Ion Channel (Vic)
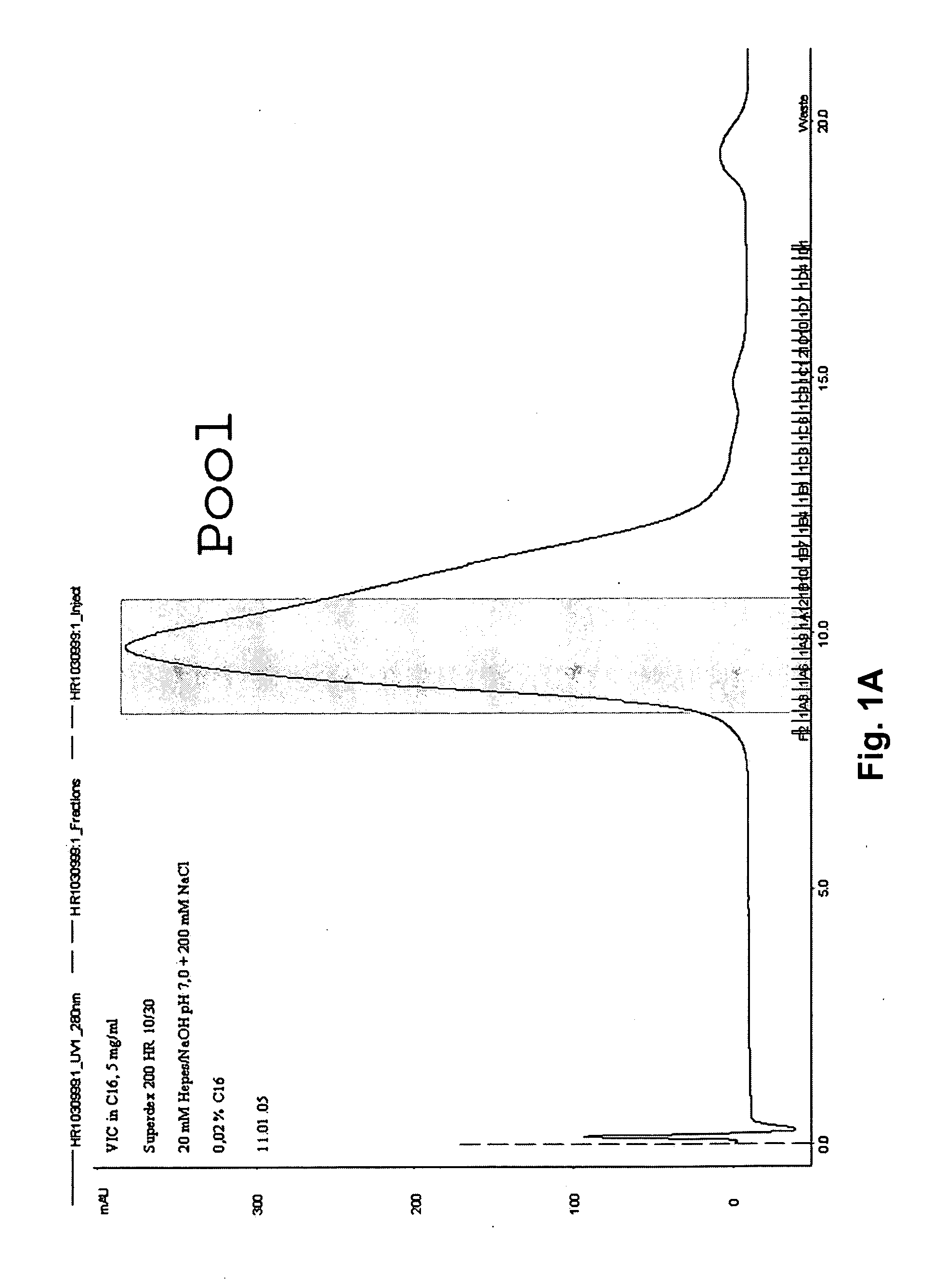
[0054] The inclusion bodies are solubilized by sonification and by the addition of solubization buffer [25 mM Tris / HCl pH 8.5, 250 mM NaCl, 1 mM DTT, 1% Alkyl(C16) phosphorylcholine (C16-FOS-Cholin) (first detergent), 0.01 mg / ml FOLCH lipid]. Thrombine is added to this solution and incubation is performed over night at room temperature to cleave of the GST part of the construct. After that, insoluble cell debris is separated from the solubilized ion channel protein by centrifugation at 4° C.
[0055] Ni-NTA-Agarose (Qiagen) equilibrated in 50 mM HEPES / NaOH pH
[0056]7.5, 250 mM NaCl, 1% C16-FOS-Cholin (first detergent) is added to the supernatant. Incubation is performed for one hour at 4° C., wherein the receptor binds to the nickel matrix. Thereafter, the nickel matrix is filled into a chromatography column, washed two times with washing buffer [50 mM HEPES / NaOH pH 7.5, 250 mM NaCl, 0.1 mg / ml FOLCH fraction 1 brain lipi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com