Method for preparing synthesis gas, method for preparing dimethyl ether using synthesis gas, and furnace for preparing synthesis gas
a dimethyl ether and synthesis gas technology, applied in the field of synthesis gas preparation, can solve the problem of slow reduction in the amount, and achieve the effect of efficient reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
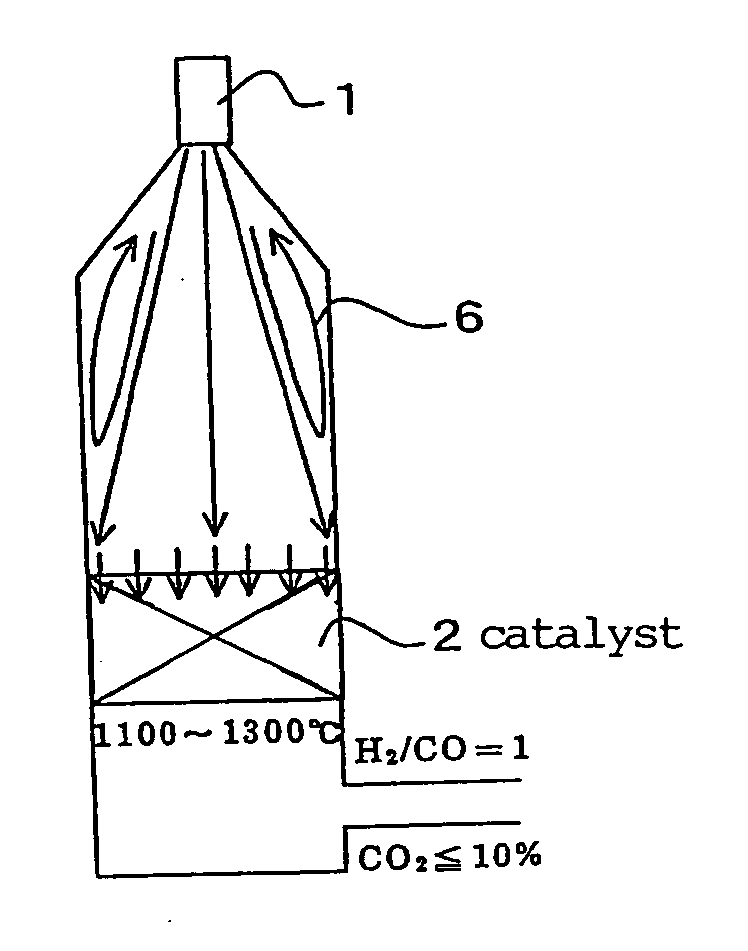
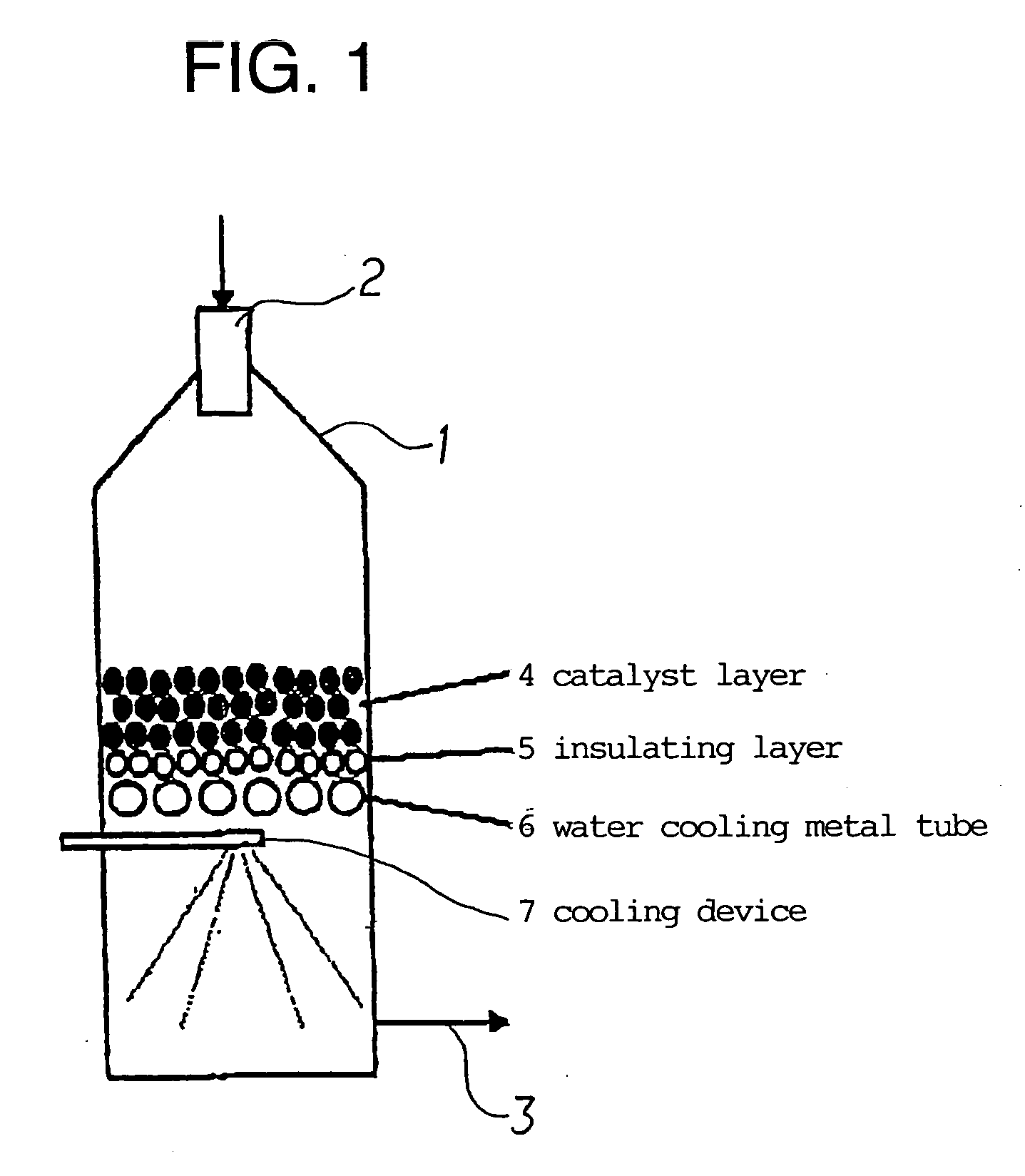
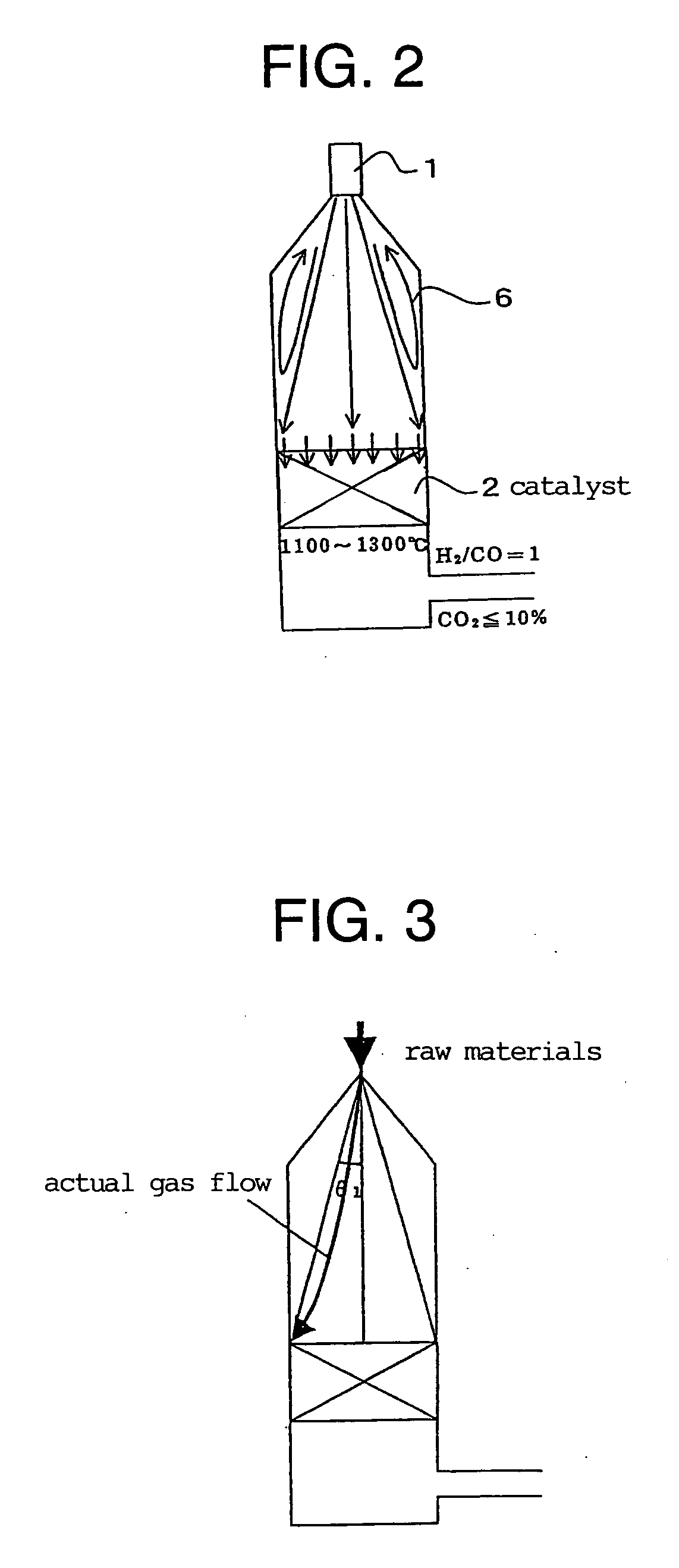
[0243] A produced synthesis gas, or a purge gas of a DME synthesis reaction system was mixed with a raw material and introduced. The condition for this embodiment was such as to prepare a synthesis gas having the H2 / CO ratio of 0.8 to 1.2. Under such condition, since the amount of the introduced steam was small, the gas excluding the condensed water was introduced. The outlet temperature of a catalyst layer was set at 1100 to 1300° C., and the synthesis gas was rapidly cooled to 600° C. or less in the lower part of the catalyst layer.
[0244] In Table 2, Comparative Examples 1 and 2 show examples of not circulating the synthesis gas, and Examples 1 and 2 show examples of circulating the synthesis gas.
[0245] It was found that in Examples 1 and 2, the amount of the introduced CO2 in the raw gas could be lowered, and also the amount of CO2 in the produced gas was decreased to 8 dry vol %. Since it is not easy to reduce the amount of CO2 in a produced gas by even 1 dry vol %, the signif...
example 1
[0390] {Purification of the Medium Oil of the Present Invention}
[0391] By conducting polymerization of 1-decene as a raw material in the presence of an aluminum chloride catalyst and water as an a promoter at −20 to 30° C. and saturation with addition of hydrogen then purification, obtained was a product, that is, the medium oil. Physical properties thereof were adjusted depending on the composition of the raw material, the polymerization temperature and / or conditions for the purification (that is, distillation). The obtained medium oil was subjected to determination of chemical properties by the following means.
[0392] In particular, the weight average molecular weight of the medium oil was measured by means of mass spectroscopy and gel-permeation chromatography. The vapor pressure was determined using a boiling point procedure by means of an ebulliometer. % Cp as a percentage of paraffinic carbon atoms to the total carbon atoms was determined by the n-d-M method (ASTM D 3238). The...
example 2
[0397] {Synthesis of Dimethyl Ether / Methanol and Evaluation of Synthesis Efficiency}
[0398]FIG. 14 shows a constructional scheme of a synthesis apparatus illustrating the synthesis of dimethyl ether.
[0399] In a reactor of the synthesis apparatus, added were 1552 g of the medium oil of Example 1, then 259 g of a methanol synthesis catalyst based on copper-zinc-alumina (CuO / ZnO / Al2O3:31 / 16 / 53) and 129 g of an alumina based methanol dehydration / shift catalyst (CuO / SiO2.Al2O3) to form a slurry-bed [weight ratio of the methanol synthesis catalyst and the methanol dehydration / shift catalyst (methanol synthesis catalyst:methanol dehydration / shift catalyst)=2:1, and the total weight of the methanol synthesis catalyst and the methanol dehydration / shift catalyst being 388 g], a slurry was prepared by mixing the above and then the reactor was closed. By passing the raw gas material [carbon monoxide: 18.11 NL / min, hydrogen gas: 18.11 NL / min, the amount to be passed was determined using a mass f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| retention time | aaaaa | aaaaa |
| gas retention time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com