Immunogenic compositions for gram positive bacteria such as streptococcus agalactiae
a technology of gram positive bacteria and compositions, which is applied in the direction of antibacterial agents, immunological disorders, antibody medical ingredients, etc., can solve the problems of high management costs and considerable morbidity and mortality, and achieve the effect of improving immunogenic compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Binding of an Adhesin Island Surface Protein, GBS 80, to Fibrinogen and Fibronectin
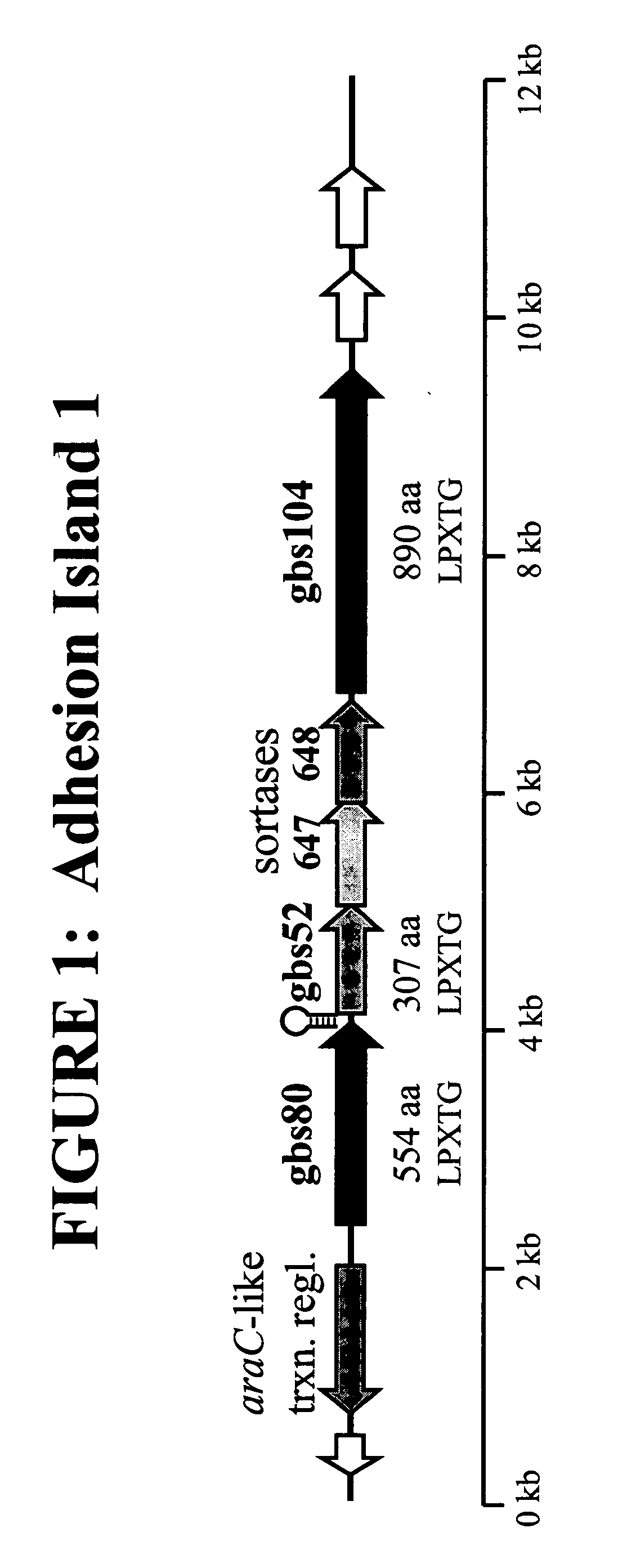
[1571] This example demonstrates that an Adhesin Island surface protein, GBS 80 can bind to fibrinogen and fibronectin.
[1572] An enzyme-linked immunosorbent assay (ELISA) was used to analyse the in vitro binding ability of recombinant GBS 80 to immobilized extra-cellular matrix (ECM) proteins but not to bovine serum albumin (BSA). Microtiter plates were coated with ECM proteins (fibrinogen, fibronectin, laminin, collagen type IV) and binding assessed by adding varying concentrations of a recombinant form of GBS 80, over-expressed and purified from E. coli (FIG. 5A). Plates were then incubated sequentially with a) mouse anti-GBS 80 primary antibody; b) rabbit anti-mouse AP-conjugated secondary antibody; c) pNPP colorimetric substrate. Relative binding was measured by monitoring absorbance at 405 nm, using 595 nm as a reference wavelength. FIG. 5b shows binding of recombinant GBS 80 to immobilized ECM...
example 2
GBS 80 is Required for Surface Localization of GBS 104
[1574] This example demonstrates that co-expression of GBS 80 is required for surface localization of GBS 104.
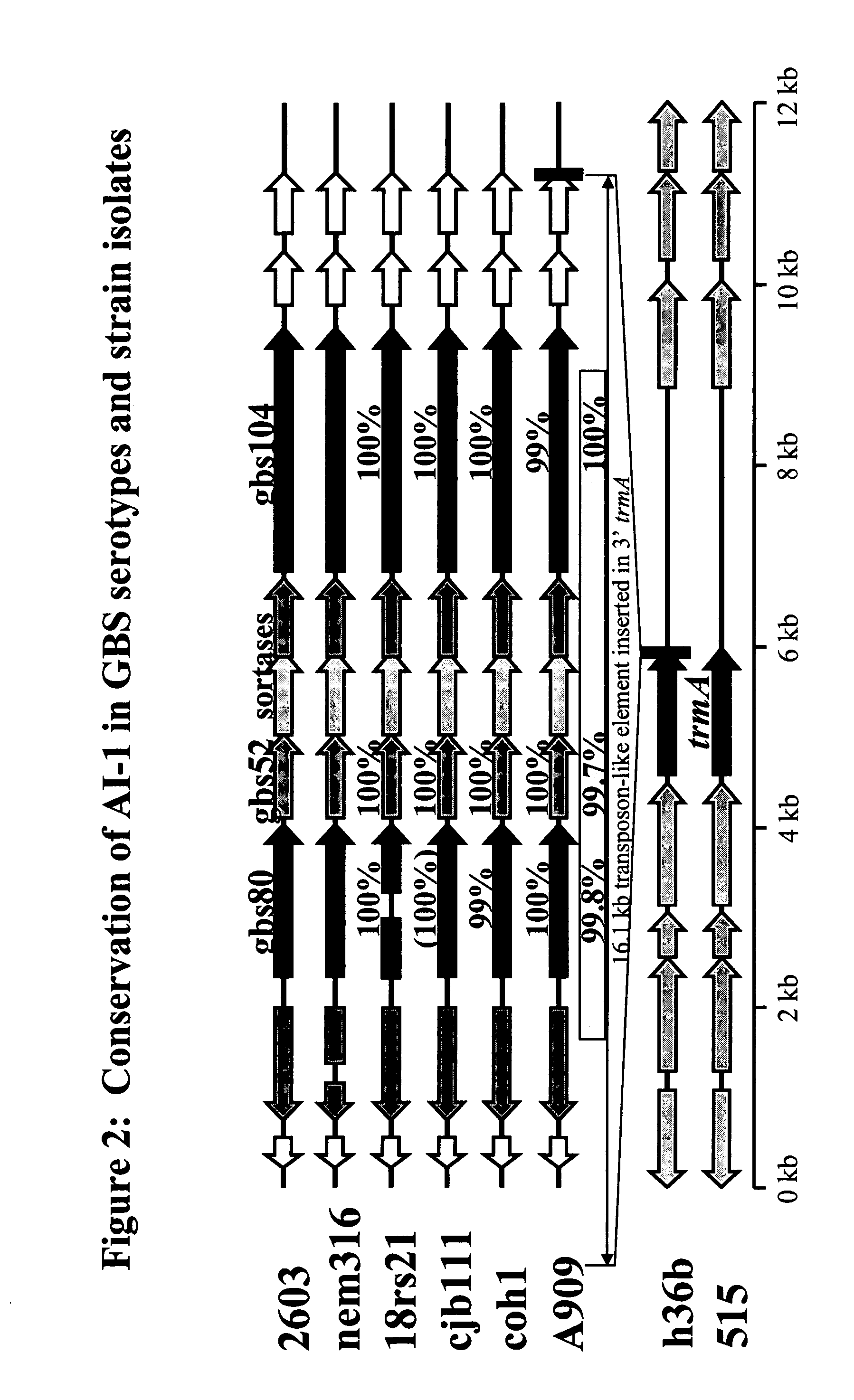
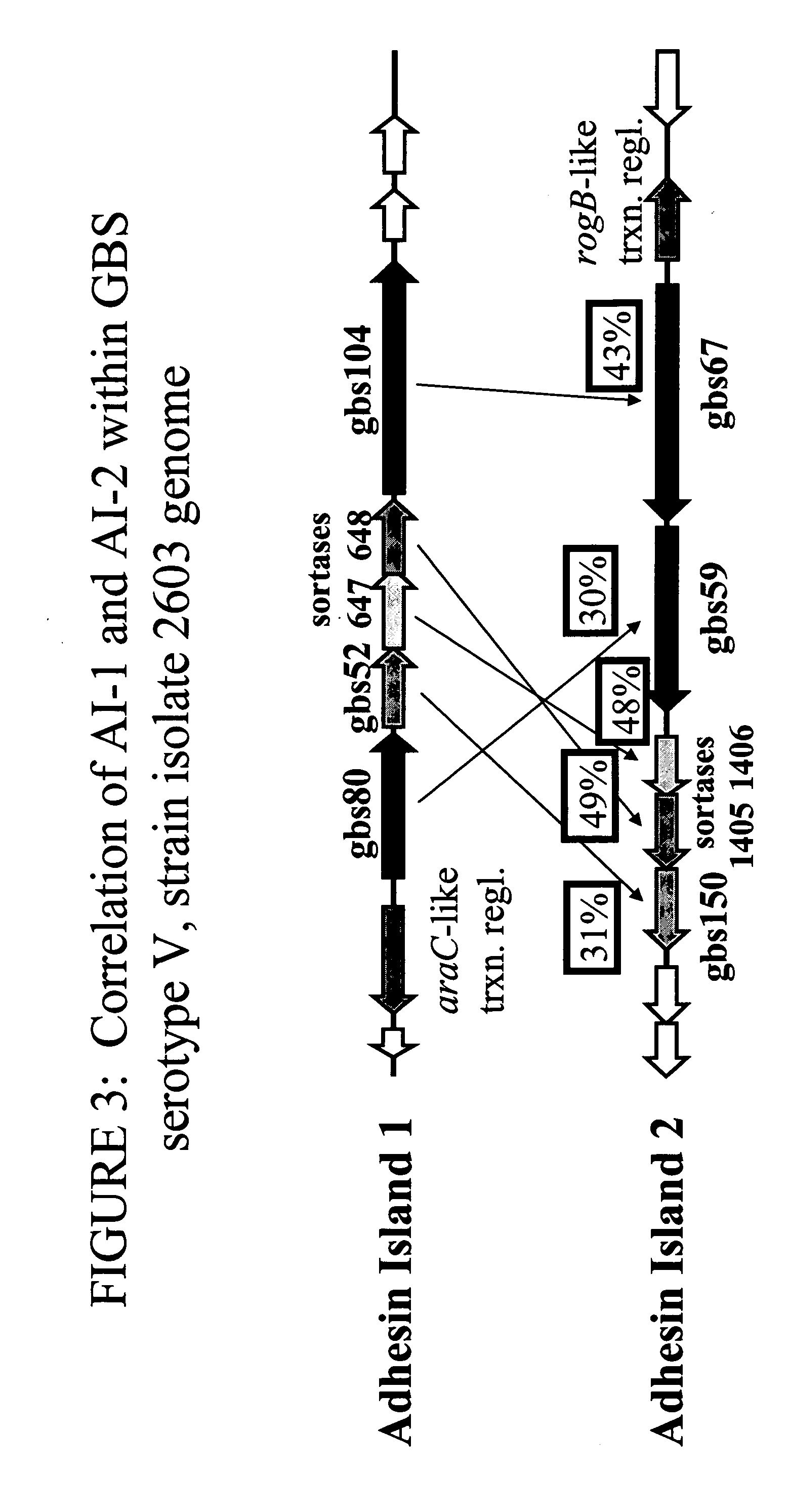
[1575] The polycistronic nature of the Adhesin Island I mRNA was investigated through reverse transcriptase-PCR (RT-PCR) analysis employing primers designed to detect transcripts arising from contiguous genes. Total RNA was isolated from GBS cultures grown to an optical density at 600 nm (OD600) of 0.3 in THB (Todd-Hewitt broth) by the RNeasy Total RNA isolation method (Qiagen) according to the manufacturer's instructions. The absence of contaminating chromosomal DNA was confirmed by failure of the gene amplification reactions to generate a product detectable by agarose gel electrophoresis, in the absence of reverse transcriptase. RT-PCR analysis was performed with the Access RT-PCR system (Promega) according to the manufacturer's instructions, employing PCR cycling temperatures of 60° C. for annealing and 70° C. for ex...
example 3
Deletion of GBS 80 Causes Attenuation In Vivo
[1588] This example demonstrates that deletion of GBS 80 causes attenuation in vivo, suggesting that this protein contributes to bacterial virulence.
[1589] By using a mouse animal model, we studied the role of GBS 80 and GBS 104 in the virulence of S. agalactiae.
[1590] Groups of ten outbred female mice 5-6 week weeks old (Charles River Laboratories, Calco Italy) were inoculated intraperitoneally with different dilutions of the mutant strains and LD50 (lethal dose 50) were calculated according to the method of Reed and Muench [Reed, L. J. and H. Muench (1938). The American Journal of Hygiene 27(3): 493-7]. As presented in the table below the number of colony forming units (cfu) counted for both the Δ80 and the Δ80, Δ104 double mutants is about 10 fold higher when compared to the wild type strain suggesting that inactivation of GBS 80 but not GBS 104 is responsible for an attenuation in virulence. This finding indicates that GBS 80 gene ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| immunogenic composition | aaaaa | aaaaa |
| surface antigen | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com