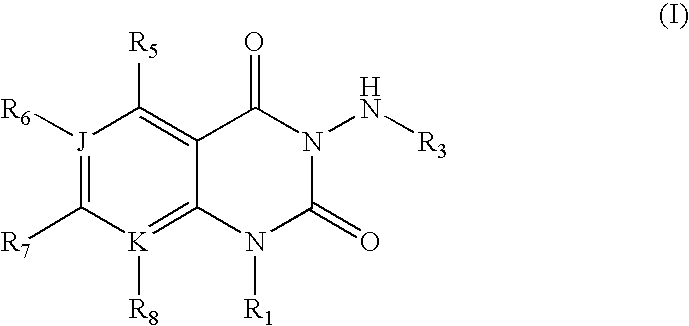
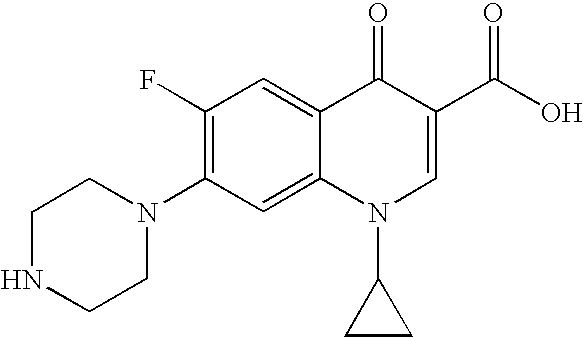
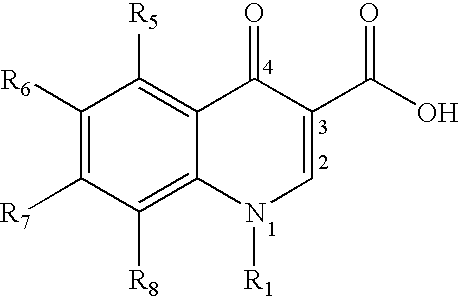
3-aminoquinazolin-2,4-dione antibacterial agents
a technology of aminoquinazolin and antibacterial agent, which is applied in the field of 3 aminoquinazolin2, 4diones, and can solve problems such as cross-resistantness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Benzoates
(a) 2,3,4,5-Tetrafluorobenzoic acid ethyl ester
[0531]
[0532] A solution of 2,3,4,5-tetrafluorobenzoic acid (4.68 g, 24.1 mmol) in dichloromethane (50 mL) at 0° C. is treated with oxalyl chloride (11.5. mL, 132 mmol) followed by N,N-dimethylformamide (4 drops). The mixture is stirred at 0° C. for 5 minutes and then at room temperature for 1.5 hours. The mixture is concentrated to dryness and subsequently co-evaporated from benzene. The resulting acid chloride is diluted with dichloromethane (50 mL), cooled to 0° C., and anhydrous ethanol (15.0 mL, 256 mmol) is added dropwise. The resulting solution is stirred at room temperature for 4.5 hours, then poured into saturated NaHCO3 and extracted with chloroform. The combined organic extracts are washed with brine, dried over Na2SO4, filtered, and concentrated under vacuum to afford the title compound (5.37 g). 1H NMR (CDCl3): δ 7.67-7.55 (m, 1H), 4.42 (q, 2H), 1.41 (t, 3H).
(b) 2,3,4,6-Tetrafluorobenzoic acid ethyl ...
example 2
Synthesis of 4-Heterocyclic Benzoates
General Procedure
[0552] A mixture of 4-fluoro substituted benzoic acid ester (1 eq.), substituted pyrrolidine (1.3-2.5 eq.), and triethylamine (2-10 eq.) in N,N-dimethylacetamide or acetonitrile is heated to 50° C.-100° C. for 15 to 60 hours. The solution is then concentrated to near dryness, poured into saturated NaHCO3 solution, and extracted with ethyl acetate. The combined organic extracts are washed with brine, dried over Na2SO4, filtered, and concentrated in vacuo. The resulting residue is purified by column chromatography (ethyl acetate / hexanes) to afford 4-pyrrolidinyl substituted benzoic acid esters.
(a) 4-[(S)-3-tert-Butoxycarbonylaminopyrrolidin-1-yl]-2,3,5-trifluorobenzoic acid ethyl ester
[0553]
[0554] A solution of 2,3,4,5-tetrafluorobenzoic acid ethyl ester (Example 1a, 4.03 g, 18.1 mmol), (S)-pyrrolidin-3 -ylcarbamic acid tert-butyl ester (4.02 g, 21.6 mmol) (Sanchez J. P., Domagala J. M., Heifetz C. L., Priebe S. R., Sesnie J. ...
example 3
Synthesis of 2-Substituted-Amino Benzoates
General Procedure
[0579] A solution of 4-(pyrrolidin-1-yl)-2-fluorobenzoic acid ethyl esters (Example 2) and cyclopropylamine (10-15 eq.) in dimethyl sulfoxide and triethylamine (2-5 eq.) is heated in a sealed glass tube for 3 days at 140° C. Compressed air is blown into the mixture to remove excess cyclopropylamine. The resulting solution is concentrated under vacuum and purified by column chromatography (ethyl acetate / hexanes) to afford 4-(substituted pyrrolidin-1-yl)-2-cyclopropylamino-benzoic acid esters.
(a) 4-[(S)-3-tert-Butoxycarbonylaminopyrrolidin-1-yl]-2-cyclopropylamino-3,5-difluorobenzoic acid ethyl ester
[0580]
[0581] A solution of 4-[(S)-3-(tert-butoxycarbonylamino)pyrrolidin-1-yl]-2,3,5-trifluorobenzoic acid ethyl ester (Example 2a, 1.95 g, 5.01 mmol) and cyclopropylamine (14.0 mL, 201 mmol) in dimethyl sulfoxide (5 mL) is heated in a sealed glass tube for 44.5 hours at 100° C. Compressed air is blown into the black solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com