Metal nano particle and method for manufacturing them and conductive ink
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
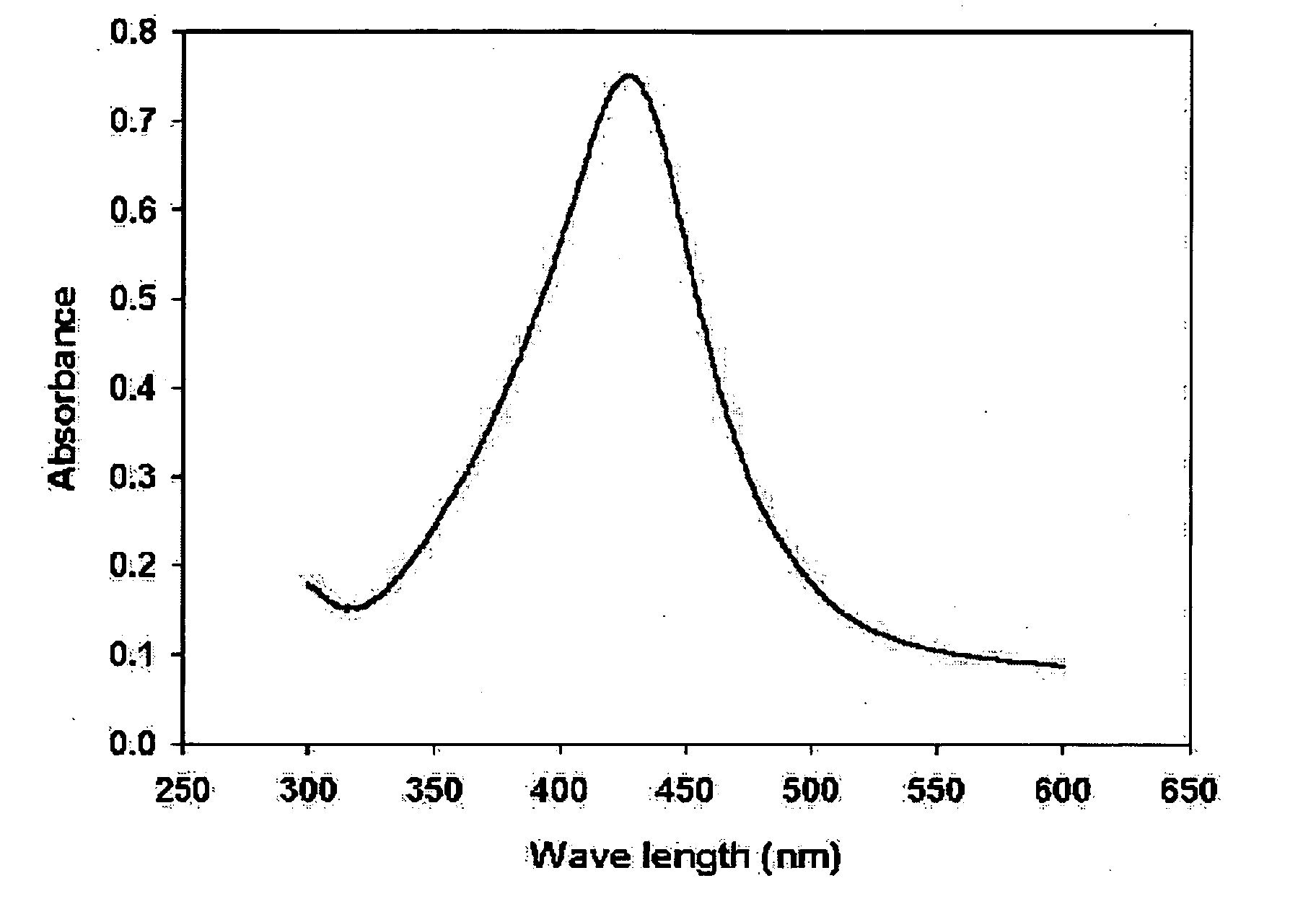
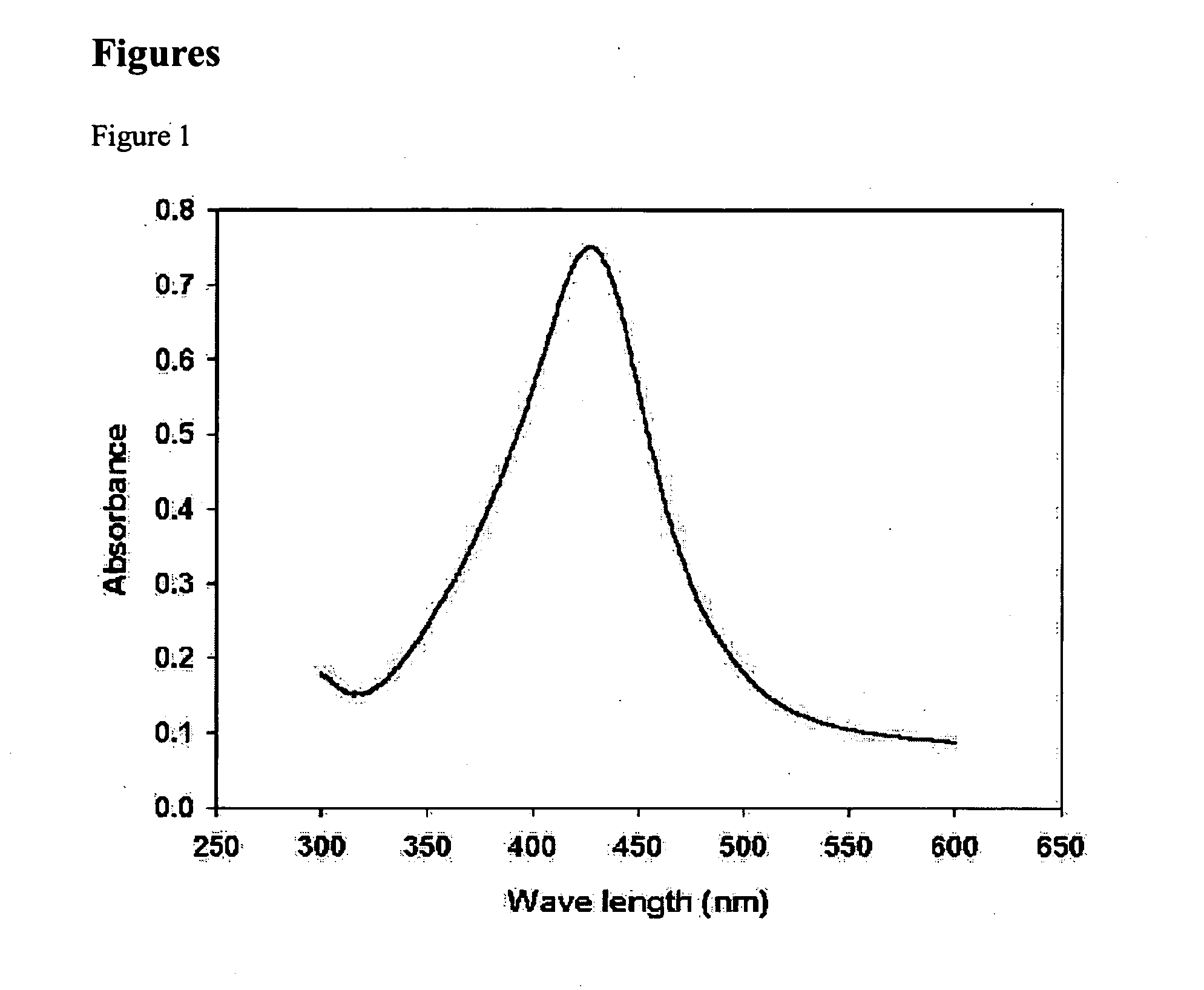
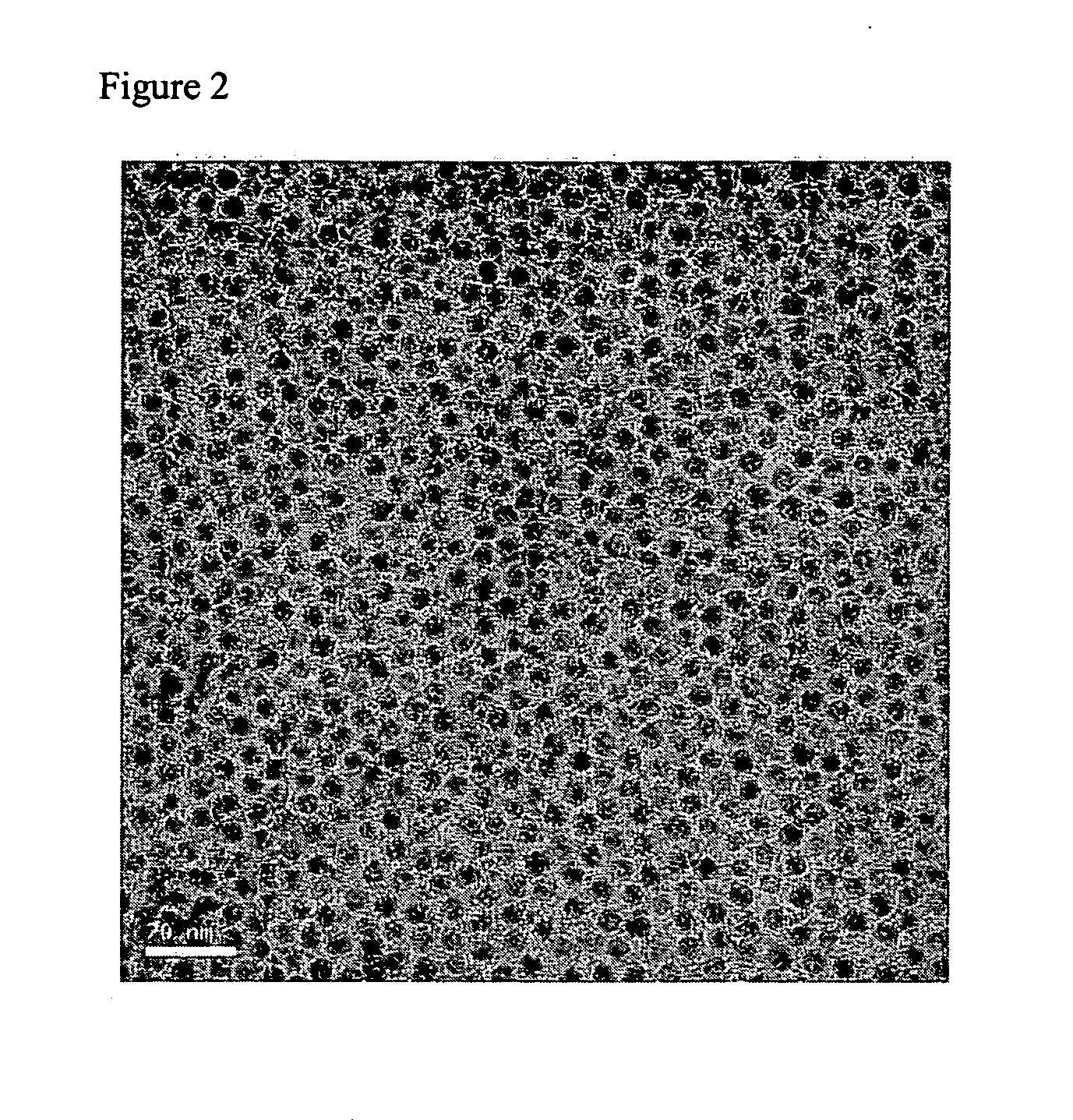
[0061] 5 g of AgNO3 was dissociated in 20 g of butylamine. The color of the solution was transparent. Here, 50 ml of toluene and 5.6 g of lauric acid were added. This mixed solution was heated to 110° C., which is the boiling point of toluene. After refluxing for 4 hours, the solution turned into a red color, and ultimately into a thick brown color. A mixture of acetone, ethanol, and methanol was added to the thick brown solution, to precipitate silver nanoparticles. These precipitates were collected after centrifugal separation. Analyzing these precipitates with a UV-VIS spectroscope provided a graph having a peak such as that in FIG. 1, by which it was found that 0.3 g of silver nanoparticles having sizes of 1 to 40 nm were obtained. From the results of TEM analysis on the particles after centrifugal separation, it was found that particles having a uniform size of 7 nm were obtained, as in FIG. 2.
example 2
[0062] 5 g of AgNO3 was dissociated in 20 g of butylamine. The color of the solution was transparent. Here, 50 ml of toluene and 5.6 g of lauric acid were added. Here, 1.6 g of TBAB was added further, which is a reducing agent. With the addition of TBAB, the color of the solution turned red. As the solution was heated up to 110° C., the boiling point of toluene, and refluxed for 2 hours, the solution gradually turned into a thick brown color. A mixture of acetone, ethanol, and methanol was added to the thick brown solution, to precipitate silver nanoparticles. After centrifugal separation of the precipitates, 1.2 g of silver nanoparticles were obtained. From the results of TEM analysis on the particles, it was found that particles having a uniform size of 7 nm were formed.
example 3
[0063] 16 g of AgNO3 was dissociated in 30 g of butylamine. The color of the solution was a faint yellow. Here, 100 g of xylene was added and the mixture stirred. Here, 20 g of lauric acid was further added, and the solution was refluxed for 20 minutes while being heated up to 140° C., the boiling point of xylene. As the reaction progressed, the solution turned into a red color, and ultimately into a thick brown color. A mixture of acetone, ethanol, and methanol was added to the thick brown solution, to precipitate silver nanoparticles. After centrifugal separation of the precipitates, 1.6 g of silver nanoparticles were obtained. From the results of TEM analysis on the particles, it was found that particles having a uniform size of 6 nm were formed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com