Method for preparing tantalum or niobium powders used for manufacturing capacitors
a technology capacitor, which is applied in the field of preparation of tantalum or niobium powder used for manufacturing capacitors, can solve the problems of large amount of remaining oxygen, difficult to control reaction, and difficult to control grain size, and achieve the effect of reducing the addition of alkaline earth metal halogen compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
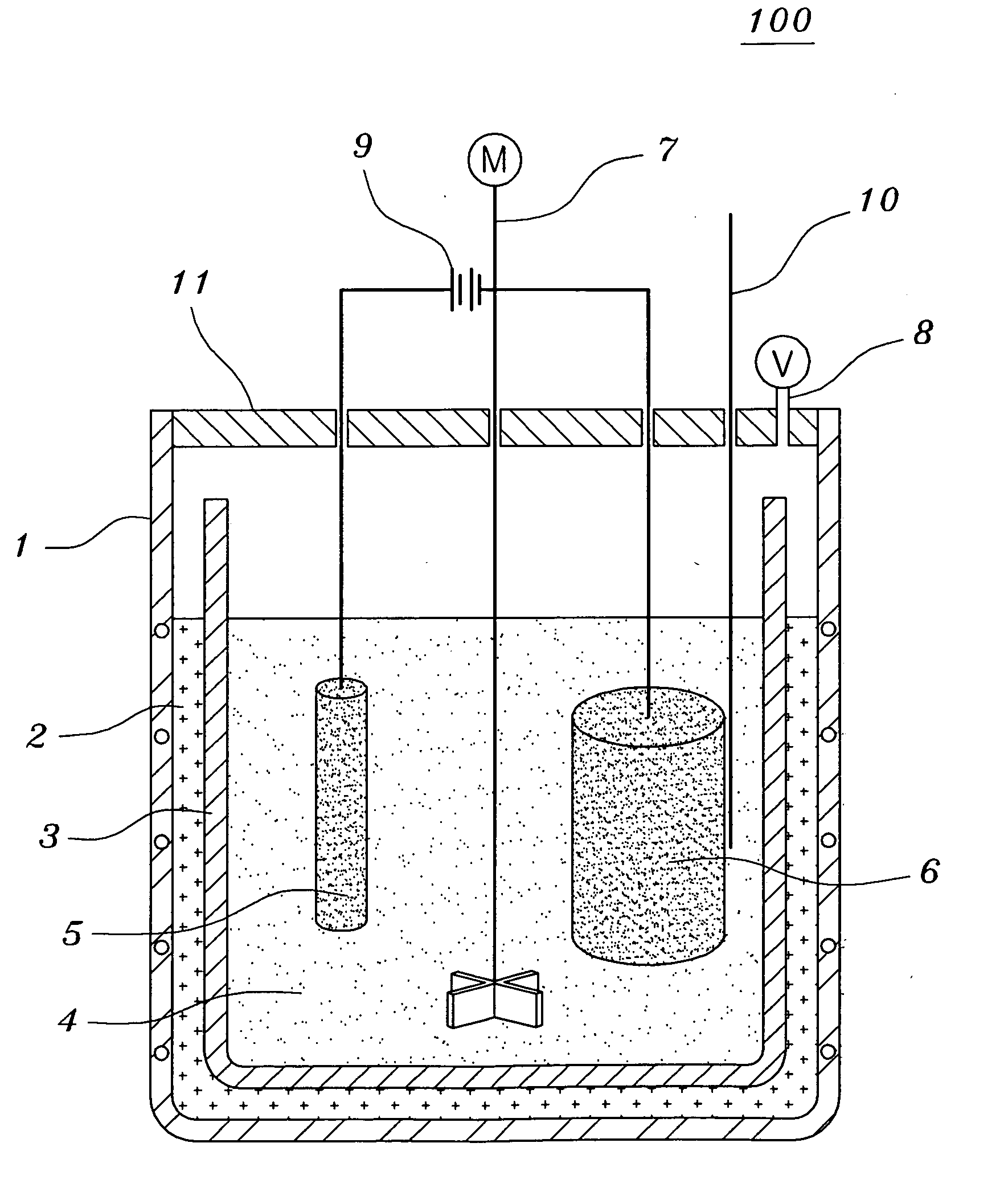
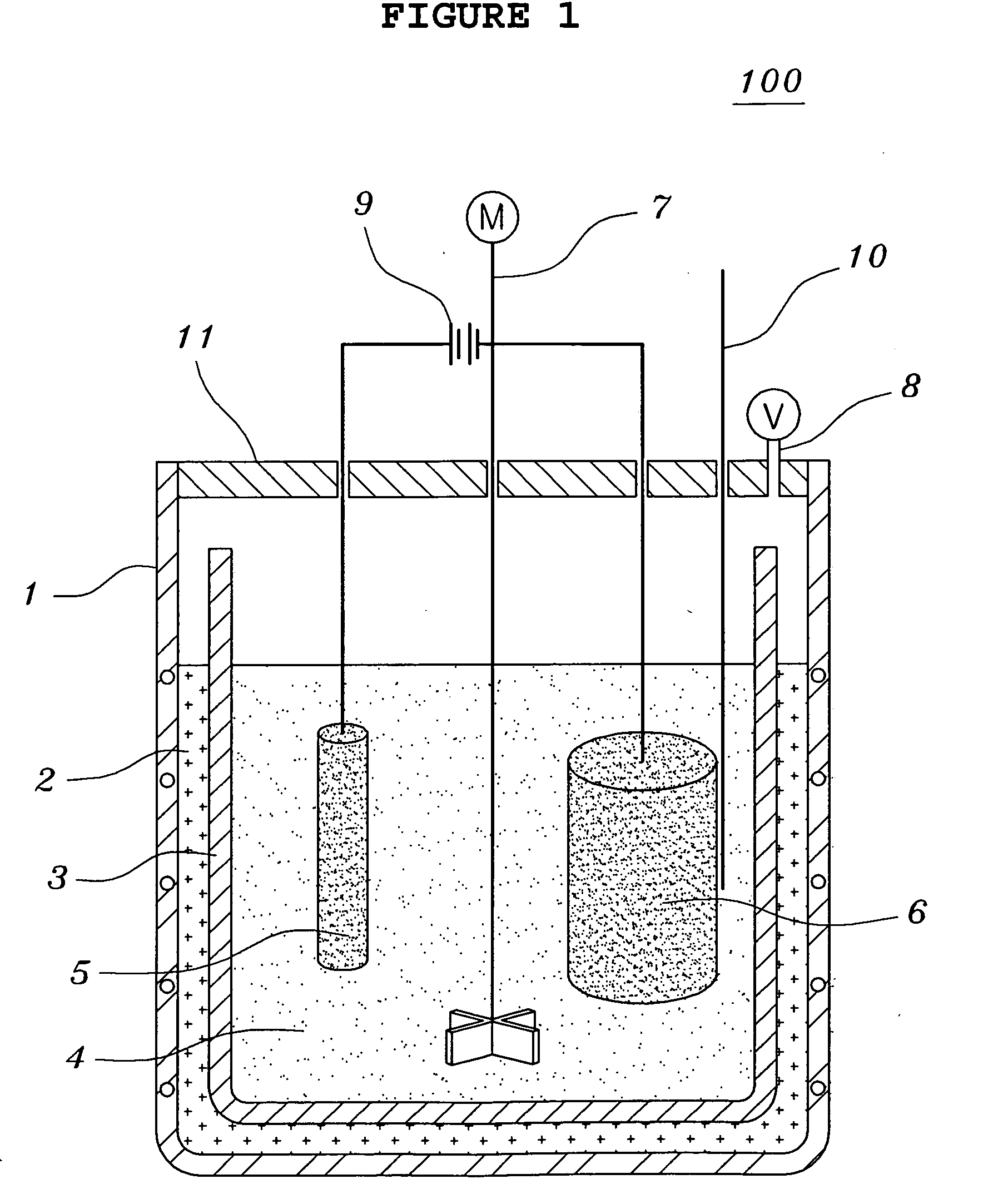
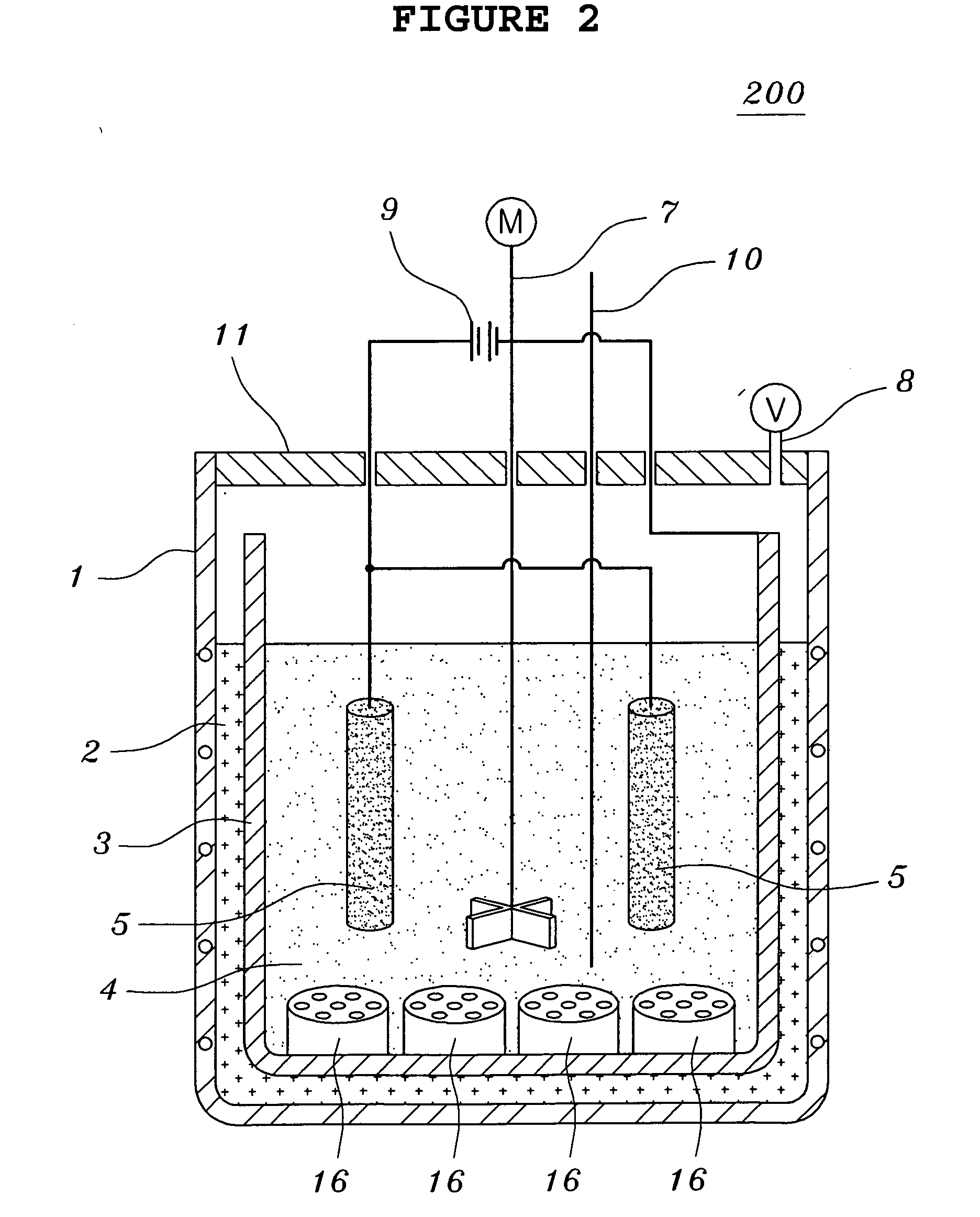
[0070]Tantalum pentoxides Ta2O5 having a content of metal impurities of 150 ppm or less and a carbon content of 10 ppm or less were used. The molten salt electrolytic reducing reactor of FIG. 1 was charged with 1 kg of LiCl and 200 g of MgCl2. 100 g of Ta2O5 having an average grain size of 0.3 μm together with steel wool were put into the cathode basket and heated at 700° C. A current was applied in order to keep a voltage of −2.8 V to −3.0 V, over the decomposition potential of MgCl2 for five hours. Here, temperature variations of the thermocouple inserted therein were observed to determine to change the voltage. That is, if a rapid increase of the temperature was observed, the voltage was decreased. After terminating the reaction, the resultant product was separated from the molten salt so as to facilitate the extraction of the product after the molten salt was cooled. The extracted product was cooled at room temperature and coarsely crushed so as to shorten the time required for ...
embodiment 2
[0074]Tantalum pentoxides Ta2O5 having a content of metal impurities of 150 ppm or less and a carbon content of 10 ppm or less were used. The molten salt electrolytic reducing reactor of FIG. 1 was charged with 1 kg of LiCl, 20.3 g of Li2O and 45 g of MgCl2. 100 g of Ta2O5 having an average grain size of 0.3 μm together with steel wool were put into the cathode basket and heated at 650° C. A current was applied in order to keep a voltage of −2.5 V to −3.0 V, over the decomposition potential of Li2O for two hours. And, a current was applied in order to keep a voltage of −2.8 V to −3.0 V, over the decomposition potential of MgCl2 for three hours. Here, temperature variations of the thermocouple inserted therein were observed to determine to change the voltage. That is, if a rapid increase of the temperature was observed, the voltage was decreased. After terminating the reaction, the resultant product was separated from the molten salt so as to facilitate the extraction of the product ...
embodiment 3
[0078]Niobium pentoxides Nb2O5 having a content of metal impurities of 150 ppm or less and a carbon content of 10 ppm or less were used. The molten salt electrolytic reducing reactor of FIG. 1 was charged with 1 kg of LiCl, 33.7 g of Li2O and 75 g of MgCl2. 100 g of Nb2O5 having an average grain size of 0.1 μm together with steel wool were put into the cathode basket and heated at 650° C. A current was applied in order to keep a voltage of −2.5 V to −3.0 V, over the decomposition potential of Li2O for two hours. And, a current was applied in order to keep a voltage of −2.8 V to −3.0 V, over the decomposition potential of MgCl2 for three hours. Here, temperature variations of the thermocouple inserted therein were observed to determine to change the voltage. That is, if a rapid increase of the temperature was observed, the voltage was decreased. After terminating the reaction, the resultant product was separated from the molten salt so as to facilitate the extraction of the product a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| conductive | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com